Left Laparoscopic Donor Nephrectomy
Abstract
Over the past decade, laparoscopic donor nephrectomy has gradually replaced the conventional open approach and has become the standard of care in living donor kidney transplantations. Compared to open nephrectomy, laparoscopic nephrectomy reduces postoperative pain, shortens the length of hospital stay, and improves the cosmetic outcome. The following illustrates our standard technique of pure laparoscopic donor nephrectomy.
Keywords
Kidney Transplantation, Laparoscopy, Donor Nephrectomy, Living Donor, Minimally Invasive Surgical Procedure
Case Overview
Focused History of the Patient
The patient is a 61-year-old female with no significant medical history other than a prior C-section. No medical history that would exclude her from donating a kidney.
Imaging
Figure 1 shows the findings from an IV contrast abdominal CT clearing the patient for left donor nephrectomy.
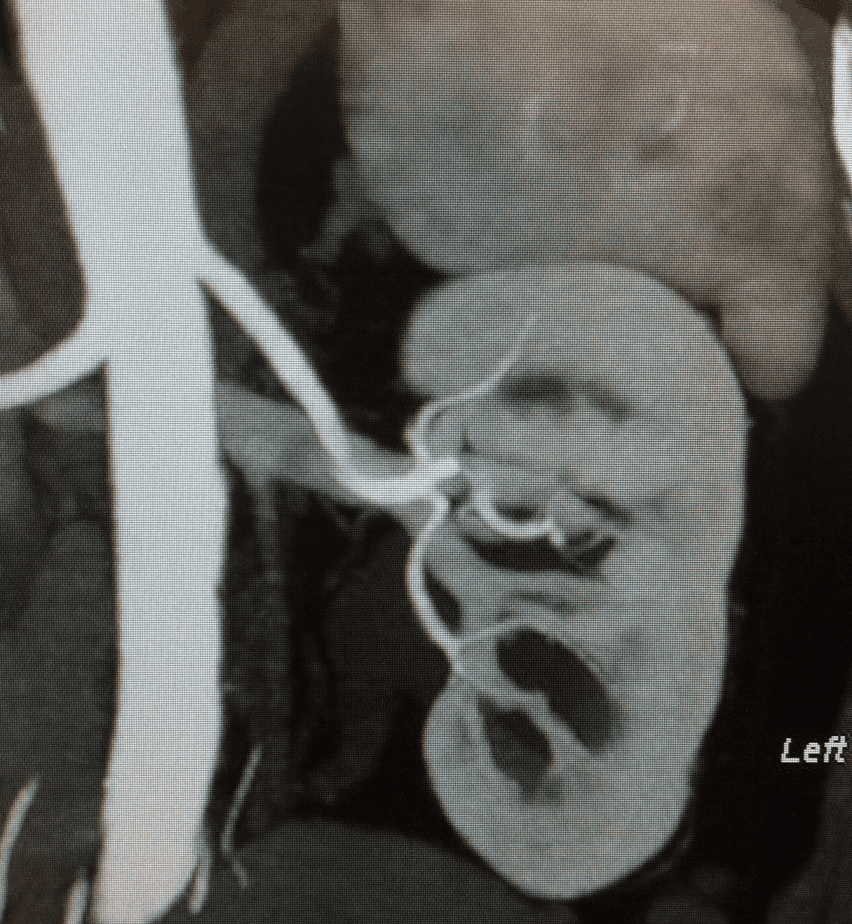 Figure 1. IV Contrast Abdominal CT. Findings: normal renal position within the renal fossae. The renal calyces are normal in morphology and distribution. Note is made of numerous left parapelvic cysts. Single renal pelvis and ureters are noted bilaterally. No pyelocaliectasis or hydroureter is present. No renal or ureteral masses or calculi are identified. RIGHT KIDNEY: 1 artery and 2 veins. LEFT KIDNEY: 1 artery and 1 vein.
Figure 1. IV Contrast Abdominal CT. Findings: normal renal position within the renal fossae. The renal calyces are normal in morphology and distribution. Note is made of numerous left parapelvic cysts. Single renal pelvis and ureters are noted bilaterally. No pyelocaliectasis or hydroureter is present. No renal or ureteral masses or calculi are identified. RIGHT KIDNEY: 1 artery and 2 veins. LEFT KIDNEY: 1 artery and 1 vein.
Step-by-Step Technique
The patient was placed on the operating room table in the right decubitus position. Incisions and port placement can be seen in Figure 2. A 6-cm long Pfannenstiel incision was made to enter the abdominal cavity. A hand port (Gelport, Applied Medical) was installed to this incision, and pneumoperitoneum was created with a pressure of 10–15 mmHg. Two 12-mm ports were inserted in her navel and left upper quadrant. A 5-mm port was also inserted in her upper abdomen on the midline.
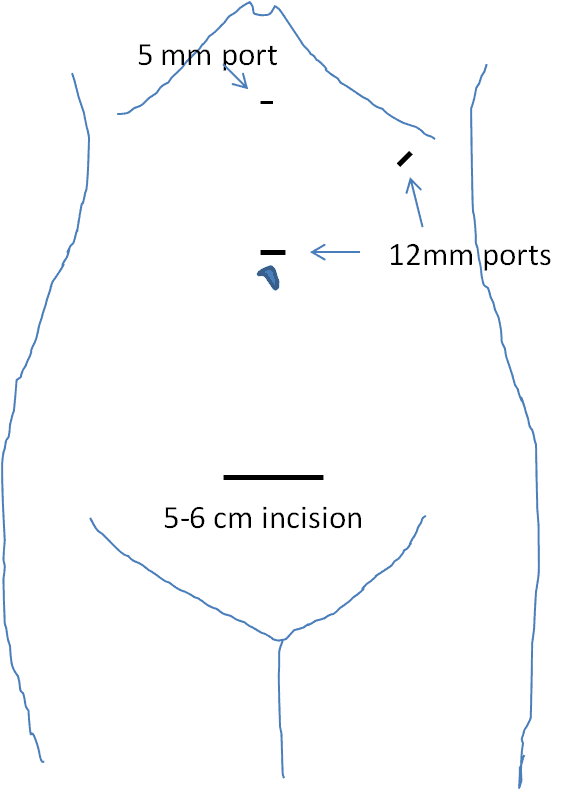 Figure 2. Incisions and port placement. A 6-cm long Pfannenstiel incision was made, and a hand port (Gelport, Applied Medical) was inserted there. Two 12-mm ports were placed in her navel and left upper quadrant. A 5-mm port was inserted in the midline of her upper abdomen.
Figure 2. Incisions and port placement. A 6-cm long Pfannenstiel incision was made, and a hand port (Gelport, Applied Medical) was inserted there. Two 12-mm ports were placed in her navel and left upper quadrant. A 5-mm port was inserted in the midline of her upper abdomen.
The procedure was started by mobilizing the left colon medially to expose the left retroperitoneal space. The left kidney was identified by dissecting the Gerota’s fascia and was then mobilized from the upper pole to the lower pole. The left ureter was then identified and freed up towards the pelvic space. To obtain better exposure of the renal hilum, a liver retractor was inserted through the Gelport. The left renal vein and gonadal vein were identified, and the gonadal vein was transected by the LigaSure. The adrenal vein was then identified, and it was also transected by the LigaSure. Next, the renal artery was identified and exposed towards the aorta. Any connective tissues or lymphatics between the renal artery and the adrenal gland or renal vein were carefully dissected. Now, the upper pole of the kidney was mobilized, and then the structure behind the renal vein was inspected by pulling up the renal pelvis. There were two lumbar veins. A smaller vein was transected by the LigaSure, and the main lumbar vein, with the diameter of approximately 5 mm, was clipped with a Hem-o-lok clip (Weck) and then transected by the LigaSure.
The kidney was completely free from surrounding structures and ready to be removed. After notifying the recipient team, the back table was set up. The ureter was clipped with a Hem-o-lok clip as distally as possible, and the ureter was transected. At this point, a 15-cm Endo Catch (Covidien-Medtronic) was inserted in the abdominal cavity through the Gelport. The left kidney was carefully placed in the bag, and the bag was closed halfway through. The kidney was then pulled laterally to stretch the artery. The renal artery was then stapled close to the aorta with ENDO TA-30 (Covidien-Medtronic), and a Hem-o-lok clip was applied over the staple. The renal artery was then transected. Next, the renal vein was stapled distal to the adrenal vein and transected. The kidney was then immediately removed from the body and perfused with a cold UW solution in the back table. Next the kidney was transported to the recipient room.
The descending colon was placed back in the original place. The two 12-mm port incisions were closed using Endo Close with 0 Vicryl suture, and the skin of those incisions were closed in one layer with 3-0 Vicryl suture. The Pfannenstiel incision was closed in four layers with 0 Vicryl suture, #1 PDS suture, 3-0 Vicryl suture, and 4-0 Monocryl suture. The patient tolerated the procedure well. TAP blocks were performed by the anesthesiologist, and the patient was transported to the recovery room in stable condition.
Discussion
More than 100,000 patients are currently on the kidney transplant waiting list in the United States, but each year there are less than 20,000 available donors (United Network for Organ Sharing, https://www.unos.org). To increase the potential donors, a minimally-invasive laparoscopic donor surgery has been developed, which has significantly increased the number of living donor kidney transplantations.1 Over the past decade, laparoscopic donor nephrectomy has gradually replaced the open nephrectomy in most transplant centers in the United States. There are no significant differences between laparoscopic and open nephrectomies with respect to graft survival at 1 year, despite the increased WIT and longer operating time associated with the laparoscopic surgery.2–4 The advantages of laparoscopic donor nephrectomy include a smaller incision, better cosmetic outcome, lower incidence of incisional hernia and adhesion, less postoperative pain, shorter hospitalization, and earlier return to work.5 Our center previously reported a retrospective single-center review to compare laparoscopic and open living donor nephrectomy.6 There was no statistically significant difference between the two groups regarding operating time, the donor and recipient’s postoperative kidney function, and the incidence of major complications. However, the length of hospital stay was significantly shorter (p < 0.0001) in the laparoscopic nephrectomy (2.87 days) than in open nephrectomy (3.6 days). When the first 100 laparoscopic cases were compared to the last 100 cases, there was a statistically significant difference in operating time in favor of the later laparoscopic nephrectomy, indicating that the laparoscopic surgery takes longer to be proficient.
In this article, we have introduced our method of performing a pure laparoscopic donor nephrectomy. However, there are two other methods of laparoscopic surgeries: hand-assisted and robot-assisted laparoscopic nephrectomies. Hand-assisted laparoscopic donor nephrectomy has been demonstrated to be advantageous in regards to the duration of the operation and the intraoperative bleeding, compared to pure laparoscopic donor nephrectomy. These advantages are most likely attributed to easier identification of the anatomy and quicker handling of any bleeding with the hand-assisted method.7–9 The experience with robot-assisted donor nephrectomy is still limited; however, it has been performed safely with favorable outcomes.9-12 Some centers reported that robotic nephrectomy is superior to laparoscopic donor nephrectomy in postoperative pain scores, analgesic requirement and length of hospital stay, despite the longer duration of the operation. However, the robotic nephrectomy is time consuming and expensive, which warrants further medical and financial analyses.
In conclusion, laparoscopic donor nephrectomy has now become the standard of care, which has the potential impact to increase the number of living donors by offering less postoperative pain, shorter length of hospital stay, and better cosmetic outcome.
Equipment
- Gelport (Applied Medical)
- LigaSure, Maryland Jaw (Covidien)
- Hem-o-lok clip (Weck)
- Multifire Endo TA 30 (2.5mm) staplers (Covidien AutoSuture)
Disclosures
Nothing to disclose.
Statement of Consent
The patient referred to in this video article has given their informed consent to be filmed and is aware that information and images will be published online.
Citations
- Ratner LE, Ciseck LJ, Moore RG, Cigarroa FG, Kaufman HS, Kavoussi LR. Laparoscopic live donor nephrectomy. Transplantation. 1995;60:1047–1049.
- Dols LF, Ijzermans JN, Wentink N, et al. Long-term follow-up of a randomized trial comparing laparoscopic and mini-incision open live donor nephrectomy. Am J Transplant. 2010 Nov;10(11):2481-7. doi:10.1111/j.1600-6143.2010.03281.x.
- Wolf JS Jr, Merion RM, Leichtman AB, et al. Randomized controlled trial of hand-assisted laparoscopic versus open surgical live donor nephrectomy. Transplantation. 2001 Jul 27;72(2):284-90. doi:10.1097/00007890-200107270-00021.
- Wilson CH, Sanni A, Rix DA, Soomro NA. Laparoscopic versus open nephrectomy for live kidney donors. Cochrane Database Syst Rev. 2011 Nov 9;(11):CD006124. doi:10.1002/14651858.CD006124.pub2.
- Fonouni H, Mehrabi A, Golriz M, et al. Comparison of the laparoscopic versus open live donor nephrectomy: an overview of surgical complications and outcome. Langenbecks Arch Surg. 2014 Jun;399(5):543-51. doi:10.1007/s00423-014-1196-4.
- Tsoulfas G, Agorastou P, Ko DS, Hertl M, Elias N, Cosimi AB, Kawai T. Laparoscopic vs open donor nephrectomy: lessons learnt from single academic center experience. World J Nephrol. 2017 Jan 6;6(1):45-52. doi:10.5527/wjn.v6.i1.45.
- Kokkinos C, Nanidis T, Antcliffe D, Darzi AW, Tekkis P, Papalois V. Comparison of laparoscopic versus hand-assisted live donor nephrectomy. Transplantation. 2007;83:41–47. doi:10.1097/01.tp.0000248761.56724.9c.
- Halgrimson WR, Campsen J, Mandell MS, Kelly MA, Kam I, Zimmerman MA. Donor complications following laparoscopic compared to hand-assisted living donor nephrectomy: an analysis of the literature. J Transplant. 2010;2010: 825689. doi:10.1155/2010/825689.
- Serrano OK, Kirchner V, Bangdiwala A, et al. Evolution of living donor nephrectomy at a single center: long-term outcomes with 4 different techniques in greater than 4000 donors over 50 years. Transplantation. 2016 Jun;100(6):1299-305. doi:10.3390/jcm10061195.
- Giacomoni A, Di Sandro S, Lauterio A, et al. Robotic nephrectomy for living donation: surgical technique and literature systematic review. Am J Surg. 2016 Jun;211(6):1135-42. doi:10.1016/j.amjsurg.2015.08.019.
- Hubert J, Renoult E, Mourey E, Frimat L, Cormier L, Kessler M. Complete robotic-assistance during laparoscopic living donor nephrectomies: an evaluation of 38 procedures at a single site. Int J Urol. 2007;14:986–989. doi:10.1111/j.1442-2042.2007.01876.x.
- Bhattu AS, Ganpule A, Sabnis RB, Murali V, Mishra S, Desai M. Robot-assisted laparoscopic donor nephrectomy vs standard laparoscopic donor nephrectomy: a prospective randomized comparative study. J Endourol. 2015;29: 1334–1340. doi:10.1089/end.2015.0213.


