Integra Scalp Reconstruction: Addressing a Full-Thickness Scalp Defect with Exposed Calvarium Along Vertex in an Elderly Immunocompromised Patient
Abstract
Reconstruction of full-thickness scalp defects often poses various challenges depending on the complexity and characteristics of the wound as well as independent patient health factors. Despite a range of reconstructive options ranging from primary closure, adjacent tissue transfer, and autografts to free flap reconstruction, there is no universally adopted decision algorithm.
Integra, an acellular matrix composed of crosslinked bovine collagen and glycosaminoglycan covered by a silicone membrane, is widely used for scalp reconstruction and has been shown to produce excellent functional and cosmetic results.
The featured case involves staged scalp reconstruction utilizing the Integra bilayer matrix wound dressing for an elderly immunocompromised patient presenting with two adjacent full-thickness scalp defects resulting in exposed calvarial bone over the vertex. The discussion centers on determining the most optimal scalp reconstructive option and exploring the treatment algorithm used at our institution. Furthermore, application of Integra for calvarial bone coverage will be discussed.
Keywords
Integra bilayer; scalp reconstruction; vertex bone exposure.
Case Overview
Background
The scalp is a sensitive and complex region, serving as a barrier and protection for the calvarium, brain, and intracranial vasculature. Defects in this region, often due to invasive cutaneous malignancies, present challenges for reconstruction, especially when gross bone exposure is involved. Adding to the complexity are various patient-specific factors that limit the effectiveness of certain reconstruction methods.
Despite the array of available options, there is no universally accepted algorithm for scalp reconstruction. Each reconstructive method must be tailored to the individual patient for optimal long-term outcomes.
Focused History of Patient
The patient is an 84-year-old male with a medical history of multiple myeloma requiring chemotherapy and immunosuppression treatment, consisting of 50 mg cyclophosphamide and 4 mg dexamethasone on a weekly basis. The patient presented with multiple malignant skin cancers at various stages of treatment and came in for the reconstruction of two adjacent scalp defects following Mohs surgery for basal cell carcinoma. The patient has an ASA score of III due to chronic heart failure, cardiomyopathy, coronary artery disease with multiple stents, and is on rivaroxaban. For the reconstruction surgery, rivaroxaban was held for a total of 2 weeks (3 days before and 1.5 week after surgery) due to a significant history of problematic bleeding issues in the past. To optimize wound healing, in consultation with his medical oncologist, cyclophosphamide and dexamethasone were held for a total of 6 weeks (1 week before and 5 weeks after surgery).
Physical Exam
Examination of the head revealed two adjacent full-thickness skin defects. There was a 4x3-cm full-thickness defect centered along the vertex of the scalp with equivalent bone exposure. Posterolateral to the midline defect, there was another 2x3-cm full-thickness skin defect with intact periosteum and no bony exposure. The edges of both wounds were intact with minimal granulation tissue. There were numerous abnormal keratotic and plaque-like skin lesions in the surrounding scalp adjacent to the two defect sites. Some were biopsy-proven malignant lesions with plans for future Mohs surgery in a staged fashion.
Imaging
Computed tomography (CT) of the head and neck with contrast was performed for further assessment of the wound, with special attention paid to the appearance of the underlying exposed cortical bone. Imaging revealed no signs of underlying bone remodeling suggestive of the presence of residual malignancy nor did it show any cervical lymphadenopathy.
When planning for scalp reconstruction, preoperative imaging combined with a comprehensive physical exam can help assess the extent of resection (and/or need for additional resection), identify important adjacent structures and any aberrant anatomy, and clarify the reconstruction necessary. CT with contrast is the initial imaging modality and is often sufficient. If initial workup revealed full-thickness cranial bone erosion with concern for intracranial involvement, additional evaluation with magnetic resonance imaging (MRI) with contrast is beneficial to assess for dural enhancement in the underlying region, ultimately rendering the lesion unresectable. Assessment for gross involvement of the sagittal sinus is also necessary for wounds involving the vertex as this would similarly be considered unresectable. Free flap reconstruction is typically required in cases of full-thickness bone defects with exposed dura. For cases requiring free flap reconstruction, additional imaging may be needed depending on the free flap being utilized.
Natural History
Defects of the scalp most commonly arise from invasive cutaneous malignancies. Other causes of scalp defects include trauma, radiation, infection, and other types of malignancies. With the rapidly rising incidence of cutaneous malignancies in the past two decades, there is a resulting increase in the need for reconstruction.1 Additionally, the incidence of cutaneous head and neck malignancies is higher in patients over the age of 65, making this a frequent population undergoing scalp reconstruction.2
Options for Treatment
The options for scalp reconstruction are extensive. Strategies include primary closure, secondary intention, local tissue advancement, regional flaps, skin grafts, dermal substitutes, and microsurgical free tissue transfer. Reconstruction can be performed in a single or multi-staged manner and may involve a combination of methods.
Acellular dermal matrices, such as Integra, are frequently used as skin substitutes in staged reconstructions. A dermal matrix is placed during the first stage of surgery and vascular ingrowth occurs over a course of 4–6 weeks. After such time, a skin graft can be placed on top of the vascularized wound bed during the second stage surgery.
Rationale for Treatment
The goal of surgical reconstruction for any defect is to restore long-term function and cosmesis while minimizing morbidity. The scalp, aside from being a highly visible and sensitive region, provides crucial function being the only protective barrier for the underlying cranial vault and intracranial structures. Reconstruction of various areas along the scalp, such as the midline vertex, requires specific attention, as there are critical underlying intracranial vascular structures, namely the sagittal sinus.
If left untreated, calvarial vault bone can withstand exposure for an extended period of time with adequate local wound care. In patients without previous radiation history or with poor surgical candidacy due to medical comorbidities, local wound care may result in granulation tissue formation with eventual skin growth, although it will take an extended period of time depending on the size of the defect. However, in previously radiated patients, the chance of exposed bone coverage from secondary intention is often low and unreliable. Additionally, exposed bone along the calvarial vault carries deleterious risks as the exposed bone will undergo remodeling over time and can progress to chronic osteomyelitis, which may result in meningitis or other devastating intracranial complications.
For this particular patient, the primary surgical goal is to quickly provide skin coverage over the exposed calvarium with minimal anesthesia time, given the patient's classification as a high-risk anesthesia candidate. Additionally, the patient requires prompt attention to his ongoing multiple myeloma treatment. Therefore, the application of Integra matrix was deemed the least invasive approach, requiring the shortest anesthesia time to achieve skin coverage over the exposed calvarium.
Special Considerations
When selecting the appropriate reconstructive option, various factors must be considered, including defect size and location, depth of involvement, exposure of cranial vault bone or dura, overall patient health, anesthesia risk, preoperative radiation history, postoperative radiation needs, integrity of surrounding tissue, and donor site morbidity.
Integra can be a useful treatment method when adequate periosteum is not available to cover the cranial vault bone, especially if adjacent scalp skin or pericranial flap is unreliable or unavailable. It can be used for medium to large scalp defects in elderly individuals with extensive medical comorbidities who may not tolerate lengthy surgeries. Ideally, it is suitable for patients without prior radiation history to the wound bed and no anticipated need for postoperative radiation therapy. However, it is not recommended for cases with exposed dura, such as in full-thickness cranial vault bone defects. In patients with exposed dura, vascularized tissue coverage such as regional or free flap reconstruction is preferred to provide more robust and reliable tissue coverage.
Surgical Procedure
This case features a patient who underwent a two-staged scalp reconstruction using Integra bilayer matrix wound dressing placement. In the first surgery, Integra bilayer matrix was applied. The second surgery was performed 6 weeks later where a skin graft from the thigh was placed.
First, the wound bed was thoroughly examined taking note of size and depth of defect, exposure of any underlying structures (calvarial bone, dura, vasculature), quality of the surrounding tissue, and the presence of any additional abnormalities (suspicious lesions). The wound edges were freshened and sharply debrided until healthy bleeding skin was encountered while removing any granulation tissue. Note that this increased the size of the defect. Thus, final sizing should not take place until the wound bed is fully prepared and the margins are cleared of malignancy. The debrided wound edges were sent for frozen pathology to evaluate for inadequate resection.
Exposed cranial vault bone was then sharply debrided by drilling down with a cutting burr to remove any overlying bacterial biofilm, to promote neovascularization into the wound bed, and to provide additional assurance of an adequate deep margin. Occasionally, methylene blue dye can be used to ink the surface of the outer cortex layer, which is then subsequently debrided until no visible dye remains at the level of the outer cortical bone. The bone was drilled until petechial bleeding from healthy bone was encountered. Care should be taken while drilling to achieve as smooth and flat of a surface as possible to avoid any contouring defects and sharp bony ledges that may extrude. At this time, the final wound defect was measured. An appropriately-sized Integra bilayer matrix sheet was then brought into the field. The sheet was then placed onto the wound bed, ensuring that the silicone side (side with black lines) was facing upwards, and subsequently trimmed to size. Note that the entire sheet needs to contact the wound bed without any wrinkles, bubbles, or gaps for optimal result. Attempts to lift or move the sheet after placement was avoided to prevent shearing forces. The sheet was then secured in place using either suture or staples. If a single defect were to require multiple Integra sheets, 2–3-mm overlap between the sheets would be utilized to minimize gaps. Small fenestrations were then made sparsely throughout the sheet to allow for fluid egress. Topical antibiotic ointment (Bacitracin or mupirocin) was then applied on top of the silicone layer. An overlying bolster (Xeroform followed by gauze was used in this case) was then placed and secured over the Integra sheet and wound bed. The Integra and bolster were subsequently kept in place for approximately 4 weeks to allow for vascular ingrowth and wound maturation. After 4–6 weeks, the patient was taken back for the second stage reconstruction, in which the top silicone layer was removed and an autologous skin graft was placed. A new bolster was secured for 10–14 days to allow for the skin graft to take. Once the bolster was removed, the patient was instructed to apply mupirocin ointment for the next 2 weeks as the skin graft matured.
Discussion
Reconstructing full-thickness scalp defects poses unique challenges, necessitating meticulous planning to determine the optimal reconstructive approach. Primary reconstruction should minimize morbidity, aiming to provide skin coverage over the cranial vault bone and intracranial contents while restoring cosmesis. Scalp wound reconstruction has historically been an extensive process requiring significant surgery (such as requiring microvascular free tissue transfer) with associated significant primary and donor site morbidity. Thus, the option to simply utilize an off-the-shelf product, such as Integra, with comparable efficacy, is a distinct privilege for the modern reconstructive surgeon.
In a landscape offering various options and surgical techniques for a specific reconstruction problem, the art of surgery and the challenge for clinicians lie in discerning the most suitable treatment option with acceptable risk and a predictable outcome. To elaborate on this, we present a treatment algorithm for scalp reconstruction currently utilized by the senior authors (CK, TL) specializing in advanced head and neck reconstruction (Figure 1).
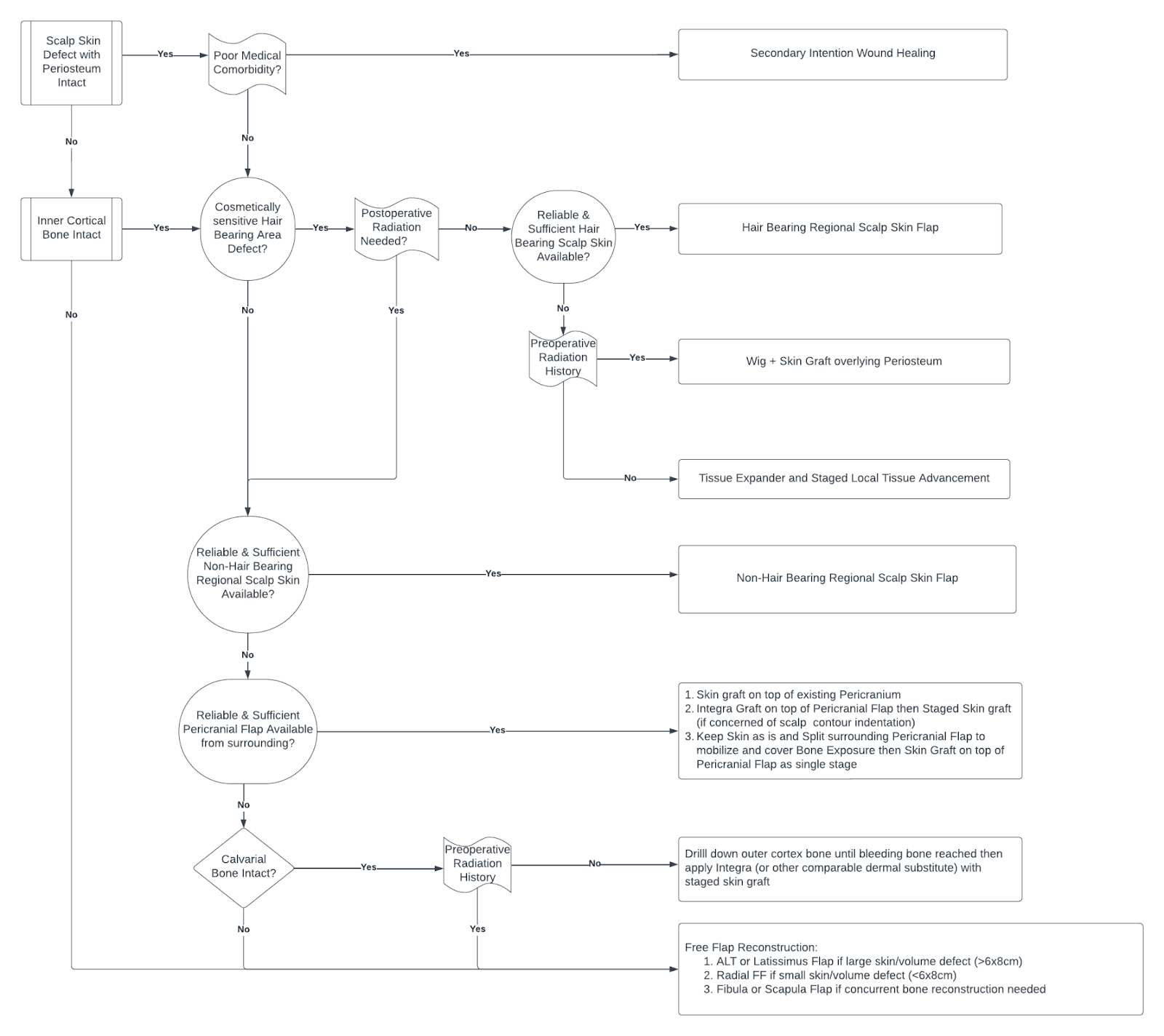
Figure 1. Treatment algorithm for scalp reconstruction.
Analyzing this treatment algorithm reveals several key factors to decision making. Determining the depth and extent of the wound defect is the first crucial step, specifically, assessing the presence of periosteum versus gross bone exposure. The presence of periosteum substantially impacts and simplifies the process as the vascularized wound bed provides a nutrient rich environment for wound healing. Resultantly, even poor surgical candidates will likely have uncomplicated wound healing with local wound care due to secondary intention healing. However, once the periosteum is violated down to cortical bone, reconstruction becomes more complicated.
In patients without periosteum and gross bone exposure, the next determining factor is cortical bone integrity and presence of exposed dura. In patients with exposed dura, there is a significantly increased risk for intracranial complications. As such, free flap reconstruction (such as anterolateral thigh or latissimus free flap) is often required to provide adequate soft tissue bulk to protect the intracranial contents. Similarly, if hardware such as a titanium mesh or polyetheretherketone (PEEK) implant is used to reconstruct the cranial bone defect, the necessity for tissue bulk arising from free flap coverage is also indicated to provide hardware coverage. However, despite the many benefits of free flap reconstruction, it also carries many pitfalls. Free flap reconstruction is significantly more technically challenging and extensive, with longer surgery times and associated higher anesthetic risks, prolonged hospital stays, and donor site morbidity. Thus, optimal surgical candidacy and the overall patient health status become of paramount significance for success.
In patients lacking periosteum with intact inner cortex of bone, regional flaps may be most reasonable. However, proceeding with regional flap reconstruction requires great caution as the success of this is highly dependent on the integrity of the surrounding tissue where the flap is generated from, which is dependent on a multitude of factors. Previous radiation history is notably one of the most important factors impacting integrity of native tissue, compromising the viability of locoregional reconstruction. Previous radiation induces irreversible tissue changes resulting from hypoxia and free radicals, creating widespread damage at the molecular level, ultimately resulting in poor wound healing mechanisms.3 Therefore, previous radiation can make both the scalp skin and underlying tissues (including the pericranium) unreliable and prone to wound breakdown. Similarly, dermal substitutes including Integra have also shown unreliable graft success in previously irradiated beds. In these cases where surrounding tissue quality is poor thereby limiting regional flap options, free flap reconstruction may be necessitated. Contrastingly, the surrounding tissue is generally favorable and regional reconstruction is typically a viable and effective option in those that have not been exposed to prior radiation therapy.
Another consideration when performing regional reconstruction is the location of the defect, more specifically in relation to the presence of hair. If the defect resides in a hair-bearing area, it is ideal to replace this with similarly hair-bearing skin to avoid a noticeable bald spot. However, one should also be conscientious of whether postoperative radiation therapy is necessitated as that would lead to hair loss and ultimately absolve the need to intentionally use a hair-bearing regional flap. In the event that hair-bearing skin is needed but not adequately available from the native surrounding scalp, one can consider the use of tissue expanders. Reconstruction would then be performed in a staged fashion, first with placement of a tissue expander to increase the area of the hair-bearing scalp followed by local tissue advancement for wound closure once sufficient expansion has occurred. Again, consideration must be taken to note previous radiation history in such cases, as tissue expander application is not advisable in case as there is inferior expandability of the irradiated skin with resulting higher risk of complication.4
There are a multitude of regional flap options available for those that are deemed an appropriate candidate. It is important to note that the surrounding scalp tissue is composed of independently vascularized layers and thus, may be raised as two separate vascularized layers if desired. It can be raised as an isolated scalp skin flap without lifting the underlying periosteum or an isolated pericranial flap without the overlying skin. In the event that the surrounding scalp skin flap is not ideal, one can consider mobilizing the underlying pericranial flap to provide vascular tissue to cover the bone defect and followed by skin graft placement on top of the pericranial flap. These two layers can also be further staggered in areas with a high risk of wound dehiscence to avoid bone exposure.
Although regional flaps are highly useful reconstruction options for scalp wounds given their high versatility, they may not be appropriate for every patient, even in cases where the surrounding tissue is appropriate and reliable. While less extensive than free flaps, they still require a fair amount of dissection with resulting operative risks, making them less favorable options for individuals with poor overall health. In these patients, less invasive techniques such as skin grafts, are good reconstructive alternatives to reduce operative times and anesthesia risks. However, the lack of a necessary vascularized wound bed (such as pericranium) often restricts their utility as an immediate reconstructive option. While there are reports of skin grafts being applied directly to bare bone, skin graft survival rates in these cases are often unpredictable and results in inadequate soft tissue coverage as well as unsatisfactory cosmesis. As such, skin substitutes and dermal regeneration matrices (Integra) present as an alternative treatment option in the reconstructive ladder and has even been regarded as ideal in this particular population.5
In the grand scheme of the treatment algorithm, Integra is best suited for patients with problematic medical comorbidities with high anesthetic risks, with poor locoregional flap options and without previous radiation history. Integra (Integra LifeScience Corporation, Plainsboro, NJ), a biologic acellular dermal matrix, has been extensively used in all forms of reconstructive procedures (burns, traumatic, excisional, malignancy, etc.) throughout the body and has shown excellent long-term functional and cosmetic outcomes in scalp reconstruction.6 It is composed of two layers: an inner layer of cross-linked bovine collagen and glycosaminoglycan covered by an outer silicone layer.7 The matrix is placed onto the wound bed and used as a temporary dressing and scaffold for tissue regeneration as host cells infiltrate the collagen matrix to form a vascularized neodermis over the course of approximately 3–6 weeks while the outer layer acts as a functional epidermis providing mechanical protection.8 Given the prolonged time necessary for cellular growth and neodermis formation, a staged reconstruction is performed. The initial stage involves Integra placement followed by a second stage of Integra silicone layer removal and skin grafting, typically 4–6 weeks later.9
A systematic review of Integra use in scalp reconstruction by Watts et al. revealed it to be widely versatile with the majority reporting an over 90% successful graft take across varying defects, including large scalp defects up to 600 cm.2 In addition, the artificial nature and readily “off the shelf” availability avoids donor site morbidity and minimizes operation time, allowing for easy and diverse application. Due to its versatility and practicality, it stands out as an ideal choice for reconstruction, especially in cases where more extensive procedures like free flap reconstruction may not be suitable. Literature reviews consistently recommend it as the treatment of choice for geriatric patients with multiple medical comorbidities.10
Despite its high versatility and success rates, Integra still carries associated disadvantages and limitations. The need for multi-stage reconstruction generates additional procedural and anesthetic risk, which may be significantly dependent on the vulnerability of the patient. However, Integra reconstruction may also be performed under sedation and local anesthesia, thereby mitigating the anesthesia risk.11 Less commonly but still with reported success, Integra may alternatively be employed in a single-stage fashion. The wound bed can be left to heal by secondary intention after removing the silicone layer 4–6 weeks postoperatively, which would negate the need for an additional procedure.12
It is important to note that providing healthy underlying vascular wound bed is critical to allow for cellular ingrowth and eventual vascularized neotissue formation.7 Defects involving calvarial exposure devoid of pericranium and fascia, which comprise the majority (up to 95%) of the reported cases of Integra scalp application, are unlikely to succeed without wound bed optimization. This most commonly entails drilling the outer cortex of the calvarium until petechial bleeding from the diploic space is visualized, encouraging vascular ingrowth from the diploe into the wound bed.10 Resulting outcomes of skin graft take with bone-burring are consistent with overall Integra success rates.7 Although bone-burring of the calvarium may be seemingly easy to perform, there are associated risks including bony contour distortion which may ultimately require additional intervention.13 The location of the defect may also increase risk of burring. For example, injury to surrounding structures at the vertex, such as the underlying sagittal sinus, may lead to catastrophic events.7,13 There have been limited reports of successful Integra application without alteration of underlying exposed calvarial bone, however the success of these cases are likely attributed to residual surrounding pericranium within the wound bed.6 Direct application of Integra onto gross bone completely denuded of periosteum has not been reported and its plausibility requires further investigation. Given the previous evidence in literature, the current recommendation is to perform bony debridement until bleeding bone is encountered to optimize Integra placement in cases of frank exposed calvarium.
Another topic highly debated with Integra application is its utility and reliability in compromised wound beds (prior radiation, surgery, scar, infection, injury). A specific area of interest is prior wound bed radiation given its fairly common occurrence as patients often have a history of multiple cutaneous malignancies. Previous research has suggested that tissue irradiation disrupts underlying cellular mechanics by reducing both the number and functional ability of cells critically involved with wound healing, ultimately compromising healing potential.10 The success of revascularization with Integra ultimately depends on host cell migration and proliferation. If these natural wound healing process are compromised, the capacity to regenerate a neovascularized tissue bed may also be reduced, potentially leading to the failure of future reconstruction with Integra.9 While there have been reported success in using Integra in previously irradiated scalp wounds, the differences in underlying variables (i.e extent of radiation and damage, wound optimization with hyperbaric oxygen) compounded with a limited sample size make it difficult to compare and produce case-controlled outcomes.14,15,16 The rates of success has also been widely variable, ranging from 50% to complete 100% graft take.11 As a result, there continues to be a lack of evidence to provide for a general consensus and recommendation in using Integra in patients with previous radiation history.
Contrastingly, in a limited number of studies, postoperative irradiation after Integra-based reconstruction has shown to be well tolerated and successful. Cases of adjuvant radiation to the reconstructed site have been reported with consistently successful percentages of graft take, with a mean in excess of 95%.9 Reported cases have also employed Integra to expedite treatment (radiation) by allowing immediate wound coverage while propagating durable tissue formation and healing through therapy. These cases have similarly reported success in skin graft application after Integra removal at the conclusion of radiation.17 While this may demonstrate the durability of Integra use in reconstructions with anticipated adjuvant radiotherapy, caution must be taken given the scarcity of reported cases. Further investigation is needed to determine its viability as a reliable standard of care in this population.
In conclusion, with the ongoing evolution of strategies and technologies for soft tissue reconstruction, we expect a continual exploration of reconstructive alternatives, particularly in the realm of dermal matrix alternatives. While Integra currently holds a high preference, the development of potentially more advanced dermal substitutes is underway. Areas of future research interest and product design may focus on implant cost reduction, antigenicity, and the feasibility of single-stage reconstruction. A novel polyurethrane biodegradable dermal substitute, known as NovoSorb BTM (PolyNovo Biomaterials Pty Ltd, Port Melbourne, Victoria Australia), is gaining traction in the literature for its use and early success in similar scalp reconstruction.18 Supported by its lower production costs, durability in infected wounds, and potentially lower infection rates, BTM shows early promise in becoming a viable, potentially even superior, option in reconstructive management.19
Equipment
- Integra® bilayer matrix wound dressing.
- Bone drill with cutting burr.
Disclosures
Nothing to disclose.
Statement of Consent
The patient referred to in this video article has given their informed consent to be filmed and is aware that information and images will be published online.
Citations
- Garcovich S, Colloca G, Sollena P, et al. Skin cancer epidemics in the elderly as an emerging issue in geriatric oncology. Aging Dis. 2017;8(5):643-661. doi:10.14336/AD.2017.0503.
- Chaiyasate K, Oliver LN, Kreitzberg SA, et al. Use of pericranial flaps with dermal substitute for scalp reconstruction: a case series. Plast Reconstr Surg Glob Open. 2020;8(8):e3011. doi:10.1097/GOX.0000000000003011.
- Miller SH, Rudolph R. Healing in the irradiated wound. Clin Plast Surg. 1990;17(3):503-508.
- Kane WJ, McCaffrey TV, Wang TD, Koval TM. The effect of tissue expansion on previously irradiated skin. Arch Otolaryngol Head Neck Surg. 1992;118(4):419-426. doi:10.1001/archotol.1992.01880040085014.
- Mogedas-Vegara A, Agut-Busquet E, Yébenes Marsal M, Luelmo Aguilar J, Escuder de la Torre Ò. Integra as firstline treatment for scalp reconstruction in elderly patients. J Oral Maxillofac Surg. 2021;79(12):2593-2602. doi:10.1016/j.joms.2021.07.009.
- Watts V, Attie MD, McClure S. Reconstruction of complex full-thickness scalp defects after dog-bite injuries using dermal regeneration template (Integra): case report and literature review. J Oral Maxillofac Surg. 2019;77(2):338-351. doi:10.1016/j.joms.2018.08.022.
- Patel S, Ziai K, Lighthall JG, Walen SG. Biologics and acellular dermal matrices in head and neck reconstruction: a comprehensive review. Am J Otolaryngol. 2022;43(1):103233. doi:10.1016/j.amjoto.2021.103233.
- Schiavon M, Francescon M, Drigo D, et al. The use of Integra dermal regeneration template versus flaps for reconstruction of full-thickness scalp defects involving the calvaria: a cost-benefit analysis. Aesthetic Plast Surg. 2016;40(6):901-907. doi:10.1007/s00266-016-0703-0.
- Johnson MB, Wong AK. Integra-based reconstruction of large scalp wounds: a case report and systematic review of the literature. Plast Reconstr Surg Glob Open. 2016;4(10):e1074. doi:10.1097/GOX.0000000000001074.
- Magnoni C, De Santis G, Fraccalvieri M, et al. Integra in scalp reconstruction after tumor excision: recommendations from a multidisciplinary advisory board. J Craniofac Surg. 2019;30(8):2416-2420. doi:10.1097/SCS.0000000000005717.
- Richardson MA, Lange JP, Jordan JR. Reconstruction of full-thickness scalp defects using a dermal regeneration template. JAMA Facial Plast Surg. 2016;18(1):62-67. doi:10.1001/jamafacial.2015.1731.
- De Angelis B, Gentile P, Tati E, et al. One-stage reconstruction of scalp after full-thickness oncologic defects using a dermal regeneration template (Integra). Biomed Res Int. 2015;2015:698385. doi:10.1155/2015/698385.
- Komorowska-Timek E, Gabriel A, Bennett DC, et al. Artificial dermis as an alternative for coverage of complex scalp defects following excision of malignant tumors. Plast Reconstr Surg. 2005;115(4):1010-1017. doi:10.1097/01.prs.0000154210.60284.c6.
- Tufaro AP, Buck DW II, Fischer AC. The use of artificial dermis in the reconstruction of oncologic surgical defects. Plast Reconstr Surg. 2007;120(3):638-646. doi:10.1097/01.prs.0000270298.68331.8a.
- Gonyon DL Jr, Zenn MR. Simple approach to the radiated scalp wound using Integra skin substitute. Ann Plast Surg. 2003;50(3):315-320. doi:10.1097/01.sap.0000046788.45508.a3.
- Chalmers RL, Smock E, Geh JL. Experience of Integra in cancer reconstructive surgery. J Plast Reconstr Aesthet Surg. 2010;63(12):2081-2090. doi:10.1016/j.bjps.2010.02.025.
- Müller CS, Jungmann J, Pföhler C, Mohammad F, Rübe C, Vogt T. Report on immediate irradiation of a rapidly growing sarcoma of the scalp prior to wound closure. J Dtsch Dermatol Ges. 2016;14(5):539-542. doi:10.1111/ddg.12786.
- Greenwood JE, Wagstaff MJ, Rooke M, Caplash Y. Reconstruction of extensive calvarial exposure after major burn injury in 2 stages using a biodegradable polyurethane matrix. Eplasty. 2016;16:e17.
- Patel NK, Tipps JA, Graham EM, Taylor JA, Mendenhall SD. Reconstruction of a near-total scalp avulsion with NovoSorb biodegradable temporizing matrix: pediatric case report. Plast Reconstr Surg Glob Open. 2022;10(12):e4717. doi:10.1097/GOX.0000000000004717.