Minimally Invasive Parathyroidectomy Under Local Cervical Block Anesthesia for Primary Hyperparathyroidism and Parathyroid Adenoma
Abstract
With both improvement in preoperative parathyroid tumor identification and the use of intraoperative parathyroid hormone (PTH) assay, minimally invasive parathyroidectomy (MIP) is now performed more frequently in patients with primary hyperparathyroidism (pHPT) compared both historically and with cervical exploration. Still, many institutions are not familiar with performing MIP under regional or local anesthesia. We present such an operation under local cervical block anesthesia.
Case Overview
Background
About 85% of patients with primary hyperparathyroidism (pHPT) harbor a single adenoma and are cured by resection of the single lesion. The remaining patients display double adenomas (3–5%) or four-gland hyperplasia (10–15%).1 Focused minimally invasive parathyroidectomy (MIP) is now feasible under regional or local anesthesia. MIP is performed after preoperative parathyroid localization usually with high-quality sestamibi scans, ultrasonography, or four-dimensional parathyroid computed tomography (4DCT) scans. A rapid intraoperative parathyroid hormone (PTH) assay is employed to confirm an adequate resection.
Focused History of Patient & Physical Exam
The patient is a 60-year-old female with biochemically unequivocal primary hyperparathyroidism. She was being evaluated for a thyroid nodule and on work up for that was found to have elevated blood and urine calcium levels. The patient's symptoms include frank osteoporosis with a T-score of -2.6 in the femoral neck and -2.3 in the lumbar spine. She has no history of nephrolithiasis or overt neurocognitive symptoms. There are no complaints of hoarseness, difficulty swallowing, or difficulty breathing. She has no history of radiation to the neck or face.
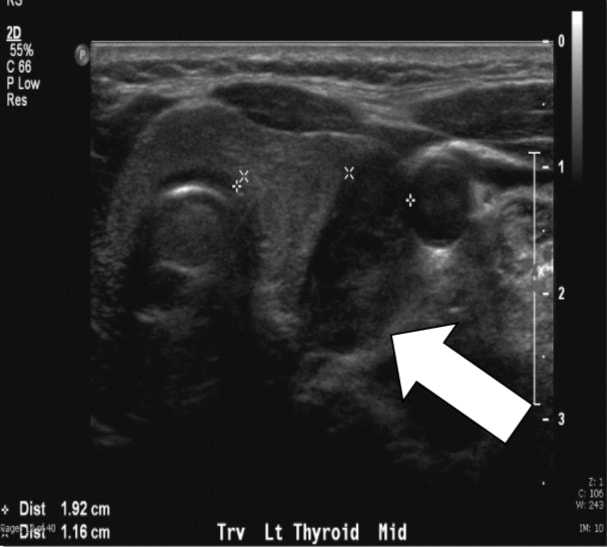
The biochemical evaluation demonstrates a total serum calcium of 10.7 mg/dl (reference range 8.8–10.2 mg/dl), elevated PTH levels of 76–81 pg/ml (reference range 10–65 pg/ml), and hypercalciuria with a 24h urine calcium of 438 mg/24h. Preoperative imaging with both ultrasound and sestamibi with single-photon emission computed tomography (SPECT) suggested a left lower parathyroid lesion.
Imaging
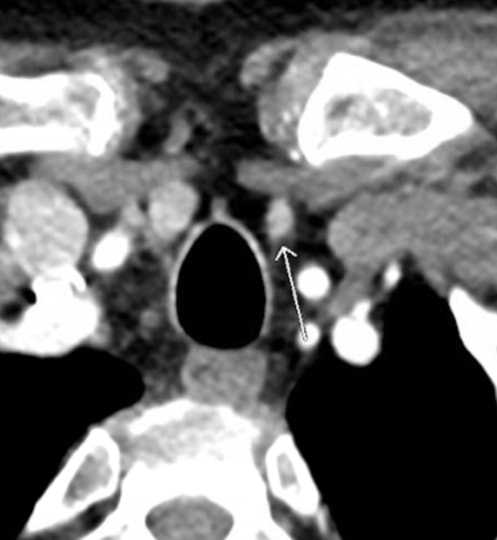
The single most accurate imaging modality for preoperative planning in patients with pHPT is the parathyroid 4DCT. The parathyroid 4DCT is similar to CT angiography.2 The term is derived from three-dimensional CT scanning with an added dimension referring to the changes in perfusion of contrast over time. Exquisitely-detailed, multiplanar images are obtained that accentuate the differences in the perfusion characteristics of hyperfunctioning parathyroid glands (eg, rapid uptake and washout) to those of normal parathyroid glands and other structures in the neck. Compared with sestamibi with SPECT, 4DCT is significantly less expensive but associated with higher exposure to ionizing radiation and thus should be used cautiously in children and young adults.3 Moreover, due to the use of intravenous contrast, it should be avoided in patients with renal insufficiency as well as in patients with a concomitant, well-differentiated thyroid carcinoma.
The most commonly used modality remains sestamibi with SPECT, which generates three-dimensional localization. A major limitation of sestamibi scans is the coexistence of thyroid nodules or other metabolically active tissues (eg. lymph nodes, thyroid nodules, and metastatic thyroid cancer) that can mimic parathyroid adenomas, thereby causing false-positive results. Sestamibi with SPECT does not provide detailed anatomical depiction and can only detect double adenomas and multiglandular hyperplasia in 25–45% of cases.2
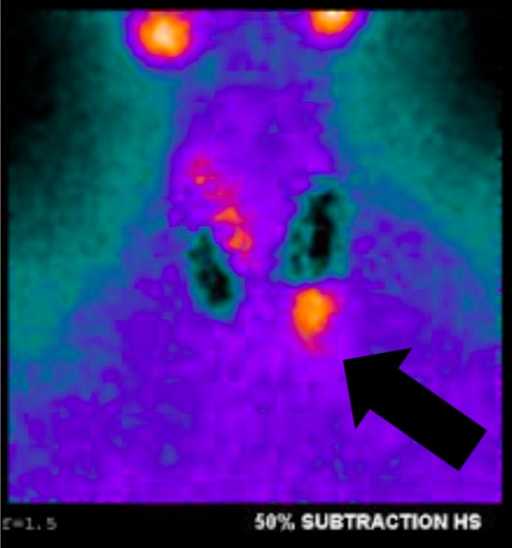 Fig. 1a, Sestamibi with SPECT of this article's patient. Arrow indicates the parathyroid adenoma in the left lower position.
Fig. 1a, Sestamibi with SPECT of this article's patient. Arrow indicates the parathyroid adenoma in the left lower position.We perform ultrasound routinely because it is effective, noninvasive, and inexpensive. The limitations include both operator dependency and inability to image mediastinal adenomas because it is limited to the neck. The normal parathyroid gland is generally too small to be visualized sonographically, whereas the parathyroid enlargement seen in pHPT is often identified as a homogeneously hypoechoic extrathyroidal ovoid mass. Parathyroid adenomas are typically vascular, and an arterial branch can often be followed to the superior or inferior pole of the lesion. By itself, ultrasound has approximately a 50–75% true-positive rate with generally better rates for larger glands.2
This patient was referred after positive imaging by the endocrinologist (both the ultrasound and sestamibi with SPECT suggested a left lower parathyroid lesion). In such a scenario, I would not subject the patient to a 4DCT scan.
Natural History
The natural history of untreated pHPT has been studied in detail and involves deteriorating bone and renal, neurocognitive, and cardiovascular functions, which are all beyond the scope of the article.4
Options for Treatment
There are no other curative treatment for pHPT except surgery. However, lowering the serum calcium temporarily can be done pharmacologically.4
Rationale for Treatment
The indications for MIP are the same as those for traditional cervical exploration: symptomatic patients or those with asymptomatic pHPT fulfilling the criteria established by the most recent National Institutes of Health (NIH) consensus meeting.4 In addition, there are now significant data to support more liberal use of surgery because the disease has been associated with several “nonclassical” morbidities, some of which seem to improve postoperatively.4 These include neurocognitive impairments and cardiovascular abnormalities.
Discussion
Unilateral surgery for pHPT was first advocated in 1975, and the side to be explored was chosen based on palpation, esophageal imaging, venography, or arteriography.5 The success of MIP has been confirmed by evidence of cure and complication rates that are at least as good as those achieved by conventional bilateral exploration.6 The complication rate of MIP is similar or lower compared with the standard cervical approach.7 Recurrent laryngeal nerve injury may occur in 0.5–1.0% of cases.6 The risk of permanent hypoparathyroidism is absent if a single gland is explored and removed, but there is always a concern in patients undergoing subtotal parathyroidectomy for multiglandular disease.
The current patient demonstrated a biochemical cure of her pHPT and had no complications.
Regional Block Anesthesia Technique
I prefer local and regional block anesthesia with monitored anesthesia care (MAC) as opposed to general anesthesia using either an endotracheal tube (ETT) or laryngeal mask airway (LMA). The regional block is performed by the surgeon in the operating room, and intravenous supplementation is directed by the anesthesiologist. In most patients, 1% lidocaine containing 1:100,000 epinephrine is used and added during the operation as required. Care is taken to aspirate before delivering the anesthetic to avoid intravascular administration. The total cumulative volume of lidocaine administered is typically 18–25 ml. Intravenous sedation is used to minimize patient anxiety while maintaining an awake, conscious patient who can phonate.1
Regional anesthesia avoids complications associated with general anesthesia, such as nausea and vomiting. Avoiding endotracheal intubation is beneficial because it has been reported to cause vocal cord changes in up to 5% of patients.6, 8 Furthermore, exploring a conscious patient permits intraoperative assessment of the superior and recurrent laryngeal nerve functions because the patient can vocalize during the procedure.
Surgical Technique and Results
No preoperative imaging modality will replace the need for a well-trained, thoughtful parathyroid surgeon.9 Surgeons performing MIP must understand the embryology and anatomy of the parathyroid glands. The embryonic development and descent into the cervical neck of the parathyroid glands lead to a highly-variable anatomy. Ectopic parathyroid tissue is commonly encountered within the thyroid, thymus, mediastinum, carotid sheath, and tracheoesophageal groove. Undescended glands can be located along the carotid bifurcation or along the larynx.
The MIP technique is individualized after the parathyroid adenoma has been localized. Typically, a 2.5–3.5-cm abbreviated Kocher incision is made, followed by the creation of limited subplatysmal flaps and the opening of the median raphe. The thyroid gland is then mobilized anteromedially. The parathyroid adenoma is then identified, aided by the preoperative imaging. It is important to handle the parathyroid adenoma gently to avoid rupture of its capsule, which may spill parathyroid tumor cells. If the parathyroid gland is grasped, it is preferable to handle the parathyroid by the fat pad often extending around the gland or its end-arterial blood supply. The end-arterial blood supply is ligated using clips or silk ties. Prior to excision of the parathyroid adenoma, the recurrent laryngeal nerve is protected. Parathyroid surgery is a meticulous procedure and operative experience correlates with rates of recurrence and persistence as well as complications.1 The procedure is guided by intraoperative PTH measurements.
Intraoperative PTH
Intraoperative PTH measurement is employed routinely. The circulating half-life of PTH is 3.5–4.0 minutes, and thus PTH levels are obtained prior to surgery and at 5 and 10 minutes after tumor extraction. PTH levels should decline (>50%) within 5 or 10 minutes after the removal of the hyperfunctioning parathyroid adenoma as the remaining normal parathyroid glands are the only source of PTH. If this is the case, the patient requires no additional exploration. Failure of the peripheral venous PTH level to adequately decline suggests remaining hyperfunctioning parathyroid tissue, and additional surgery is indicated under either regional or general anesthesia. In addition to being a valuable adjunct to confirming the completeness of parathyroid resection, the rapid PTH assay is a useful adjunct to other aspects in the treatment of pHPT. We routinely perform ex vivo fine needle aspirations of tissue excised during parathyroid surgery to measure PTH. A positive aspirate will demonstrate PTH levels greater than 1,000 pg/ml. This has eliminated the need for frozen section analysis in most cases. Although we rely heavily on the intraoperative PTH assay, it does not replace clinical judgment, and the assay should be interpreted in this context.1
Pathology and Follow-Up
The pathology revealed an enlarged (1.8 cm) and cellular parathyroid gland weighing 507 mg (normal about 30–40 mg). At the postoperative visit eight days following surgery, the patient’s total serum calcium was normal at 9.5 mg/dl (reference range 8.8–10.2 mg/dl), and she had a normal PTH level of 32 pg/ml (reference range 10–65 pg/ml). Her vocal cord function was normal as well.
Equipment
No special equipment was used.
Disclosures
Nothing to disclose.
Statement of Consent
The patient referred to in this video article has given his informed consent to be filmed and is aware that information and images will be published online.
Citations
- Carling T, Udelsman R. Focused approach to parathyroidectomy. World J Surg. 2008;32(7):1512-1517. doi:10.1007/s00268-008-9567-z.
- Starker LF, Mahajan A, Björklund P, Sze G, Udelsman R, Carling T. 4D parathyroid CT as the initial localization study for patients with de novo primary hyperparathyroidism. Ann Surg Oncol. 2011;18(6):1723-1728. doi:10.1245/s10434-010-1507-0.
- Mahajan A, Starker LF, Ghita M, Udelsman R, Brink JA, Carling T. Parathyroid four-dimensional computed tomography: evaluation of radiation dose exposure during preoperative localization of parathyroid tumors in primary hyperparathyroidism. World J Surg. 2012;36(6):1335-1339. doi:10.1007/s00268-011-1365-3.
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3561-3569. doi:10.1210/jc.2014-1413.
- Roth SI, Wang CA, Potts JT Jr. The team approach to primary hyperparathyroidism. Hum Pathol. 1975;6(6):645-648. doi:10.1016/S0046-8177(75)80073-6.
- Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253(3):585-591. doi:10.1097/SLA.0b013e318208fed9.
- Bergenfelz A, Lindblom P, Tibblin S, Westerdahl J. Unilateral versus bilateral neck exploration for primary hyperparathyroidism: a prospective randomized controlled trial. Ann Surg. 2002;236(5):543-551. doi:10.1097/01.SLA.0000032949.36504.C3.
- Kark AE, Kissin MW, Auerbach R, Meikle M. Voice changes after thyroidectomy: role of the external laryngeal nerve. Br J Med (Clin Res Ed). 1984;289(6456):1412-1415. doi:10.1136/bmj.289.6456.1412.
- Stålberg P, Carling T. Familial parathyroid tumors: diagnosis and management. World J Surg. 2009;33(11):2234-2243. doi:10.1007/s00268-009-9924-6.