Brain Biopsy of a Suspected Cerebellar Lymphoma
Abstract
In neurosurgery, brain biopsy is an essential tool for providing adequate histological sampling in neoplastic and non-tumorous lesions. There are two main techniques in obtaining tissue samples: open biopsy requiring craniotomy or needle biopsy. Needle biopsies allow for minimally-invasive tissue diagnosis with less risk of operative morbidity for the patient. Here we show a frameless needle biopsy of a cerebellar lesion using the Brainlab VarioGuide system.
Case Overview
Background
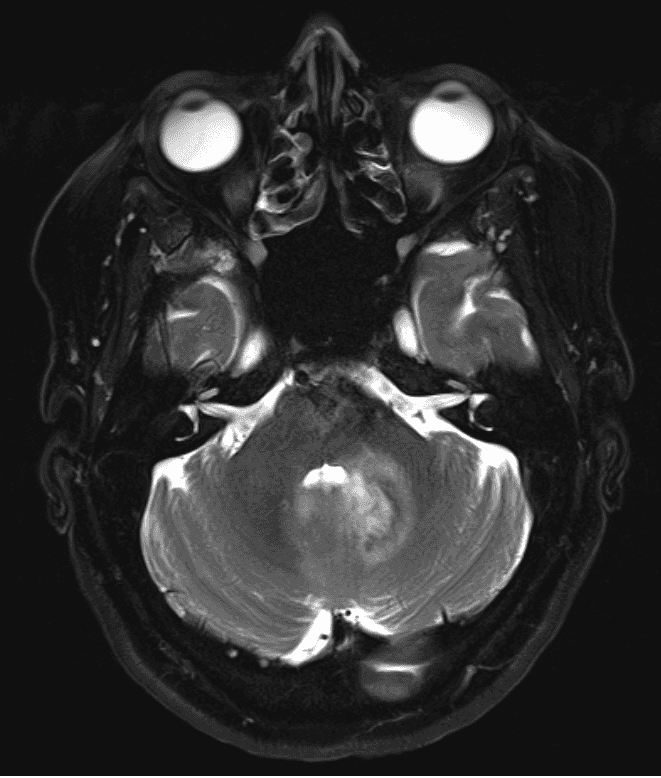
The lesion to be targeted is located adjacent to the fourth ventricle in the cerebellar parenchyma. It is not a brainstem lesion and it does not involve the cerebellar pedunculi. There is homogeneous contrast enhancement in MR imaging and some perifocal edema. No signs of hemorrhage or ischemia were detected.
Focused History of the Patient
The patient has had no severe illnesses so far, and there is no history for malignoma. Only mild arterial hypertension and mild polyneuropathy were apparent. Past immunosuppressive therapies that could augment development of lymphoma were also absent.
Physical Exam
This 72-year-old lady developed severe gait ataxia, which was so far unknown to her. There were no other cerebellar signs like dysarthria or intention tremor.
Imaging Studies
She underwent MR-imaging that showed a homogenous contrast-enhancing lesion adjacent to the fourth ventricle in the cerebellar tissue and moderate perifocal edema (Figures 1–2). Brainstem and cerebellar pedunculi were not involved. No signs of hemorrhage or ischemia were detected.
Due to the homogeneous contrast enhancement, we suspected CNS-lymphoma. Other possible diagnoses include ischemia, vasculitis, metastasis, glioma, and inflammation.
Figure 1. These gadolinium-enhanced T1 weighted images show an infratentorial lesion in the cerebellum and left cerebellar peduncle adjacent to the fourth ventricle. Please note the homogenous contrast enhancement suspicious of CNS lymphoma and missing infiltration of the brain stem.
Natural History
If untreated, this lesion would ultimately result in occlusive hydrocephalus, coma, and death. If it were to progress in size, cerebellar symptoms would increase (eg, dysarthria, intention tremor, ataxia), and brainstem symptoms are likely to worsen (cranial nerve deficits, eg, difficulties swallowing, paresis, vegetative dysregulation, coma).
Options for Treatment
Treatment options in cases of lymphoma would include chemotherapy; in glioma or metastasis, radiotherapy may be administered depending on the exact histological finding. Both possible diagnoses will require antineoplastic therapy because both tumor entities show rapid progression if left untreated. Tissue diagnosis is important to prove a tumor entity, provide molecular characterization of the lesion, and allow targeted therapies. Tissue diagnosis would be possible with open transcortical surgery, but this would require longer anesthesia time and a higher risk of cerebrospinal fluid leak and wound healing problems compared to applying a needle biopsy. In cases of lymphoma, it is important that the patient is naïve to corticosteroids before obtaining tissue samples because cortisone may prevent adequate tissue diagnosis.
Rationale for Treatment
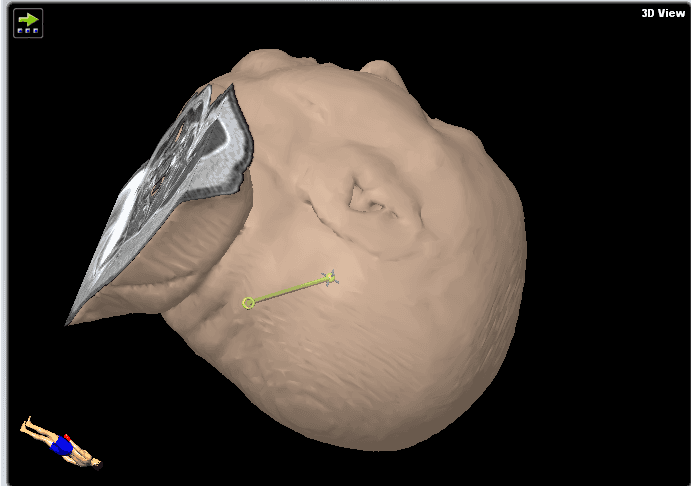
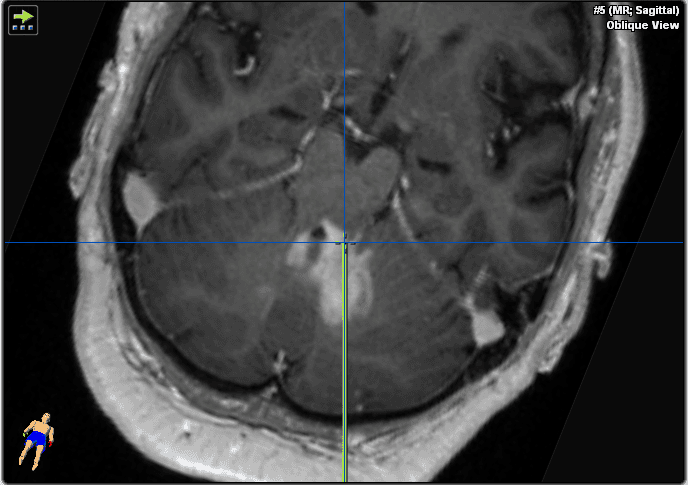
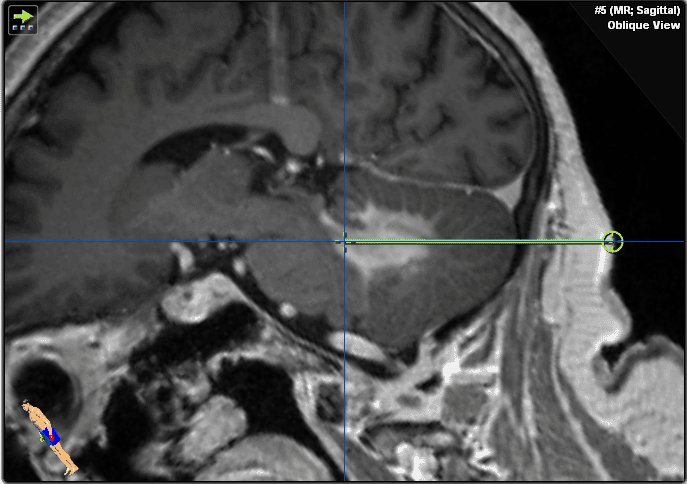
We decided to perform a brain biopsy instead of an open transcortical surgery to test for lymphoma for the reasons given in the above section (Figure 3). Neuronavigation system precision is more accurate in supine position compared to prone position due to improved visibility of face and convexity surface for the navigation camera. We therefore opted for supine position to allow maximal spatial precision in this procedure. Stereotactic biopsy would be more technically difficult due to the frame-based procedure which would require a semi-sitting position and more risk of air embolism. In purely brainstem lesions, rongeur-based stereotactic biopsy would be the more favorable technique, causing less tissue trauma (smaller biopsy needle, but also less sample volume). The floor of the fourth ventricle is to be avoided in surgical procedures due to its critical role in brainstem function.
Figure 3. These images show the plan for the trajectory.
Special Considerations
Only a few patients do not undergo tissue sampling: these are high-risk patients who are likely to die from the operative procedure or from anesthesia complications (eg, severe coronary heart disease, septic patients) or patients who will not undergo chemotherapy due to their reduced clinical status.
Discussion
We show here a procedure to allow minimally-invasive histological sampling in a posterior fossa lesion. There is a clear advantage of needle biopsy over an open procedure due to less operative complications such as cerebrospinal fluid leak and wound healing problems.9
Additionally, a needle biopsy can be obtained with the same accuracy and conclusive histological diagnosis rate as a stereotactic biopsy.1,10
Spatial precision of the navigation system is more accurate in supine position because nose, glabella, temples, and forehead are more easily accessible and may more easily be detected by the navigation system camera. In a prone position, registration of the patient may take longer, requiring more anesthesia time and may be less precise. Especially in older patients or patients with moderate-to-high anesthesiological risks, VarioGuide biopsy allows shorter operation time (Miltiadis 2017), and less procedural risk for the patient.9
Frame-based stereotactic biopsy would be more technically demanding because the stereotactic frame may not allow the desired trajectory. Frame-based stereotaxy may also require a semi-sitting position of the patient and harbor an increased risk for venous air embolism and its known complications).3,5,7,8 However, the study conducted by Nakagawa et al. on 80 patients demonstrates the safe use of suboccipital stereotactic biopsy in the supine position, with the head turned and inclined.12 In purely intrinsic anterior brainstem lesions, a rongeur-based stereotactic biopsy would be the more favorable technique as it results in less tissue trauma due to a smaller biopsy needle. However, a rongeur-based stereotactic biopsy also results in less sample volume. Lesions like the one in this case cannot anatomically be reached by this approach because rongeur-based stereotactic biopsies require a supratentorial entry point 4 cm lateral to the midline at the level of the coronal suture. Consequently, we proceeded with a needle biopsy.1,7
Critical landmarks to be avoided for bleeding complications are transverse sinus, vertebral artery, and posterior inferior cerebellar artery. We therefore chose an entry point well below transverse sinus and well above vertebral artery. Biopsy trajectory should avoid passing the brainstem (too far anterior) or the ventricle and choroid plexus (highly vascularized and increased bleeding risk). Entering the ventricle may also result in obtaining only CSF because no “suction” to the parenchyma may be applied over the self-cutting needle.
Samples from the border zone may later prove the trajectory to be precise and may allow diagnosis of a vital tumor (eg, in high grade glioma).6 Samples from the lesion core may indicate necrosis or a need to obtain more samples of the vital tumor. A vital tumor sample is essential for establishing molecular diagnosis to allow targeted therapy.11
Biopsy specimens are examined under a fluorescence light microscope to determine if the samples are from the pathologic tissue. Fluorescing samples resemble regions of disrupted blood-brain barrier and prove that the lesion was truly targeted during biopsy.2 If none of the lesions fluoresce, another biopsy trajectory could easily be added in the same operation.2
Histological sampling in this patient ruled out lymphoma and proved non-infectious rhombencephalitis. Treatment for this disease included high-dose cortisone boost therapy that led to symptom relief and improvement of MRI findings. However, the patient later developed a relapse of the disease that proved to be refractory to another cortisone therapy. Treatment was therefore changed to plasmapheresis.4
Equipment
Brainlab navigation system, VarioGuide biopsy system, and side cutting biopsy needle were used in this procedure. Preoperative trajectory planning was done using Brainlab iPlan net software.
Disclosures
No financial disclosures for any of the authors.
Statement of Consent
The patient referred to in this video article has given their informed consent to be filmed and is aware that information and images will be published online.
Citations
- Bradac O, Steklacova A, Nebrenska K, Vrana J, de Lacy P, Benes V. Accuracy of VarioGuide Frameless Stereotactic System against frame-based stereotaxy: prospective, randomized, single-center study. World Neurosurg. 2017;104:831-840. doi:10.1016/j.wneu.2017.04.104.
- Bowden SG, Neira JA, Gill BJA, et al. Sodium fluorescein facilitates guided sampling of diagnostic tumor tissue in nonenhancing gliomas. Neurosurgery. 2017. doi:10.1093/neuros/nyx271.
- Fàbregas N, Hurtado P, Gracia I, Craen R. Anesthesia for minimally invasive neurosurgery. Rev Colomb Anestesiol. 2015;43(Suppl 1):15-21. doi:10.1016/j.rca.2015.07.004.
- Fukata M, Yokoi N, Fukata Y. Neurobiology of autoimmune encephalitis. Curr Opin Neurobiol. 2017;48:1-8. doi:10.1016/j.conb.2017.07.012.
- Gracia I, Fabregas N. Craniotomy in sitting position: anesthesiology management. Curr Opin Anaesthesiol. 2014;27(5):474-483. doi:10.1097/ACO.0000000000000104.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820. doi:10.1007/s00401-016-1545-1.
- Georgiopoulos M, Ellul J, Chroni E, Constantoyannis C. Efficacy, safety, and duration of a frameless fiducial-less brain biopsy versus frame-based stereotactic biopsy: a prospective randomized study. J Neurol Surg A Cent Eur Neurosurg. 2017. doi:10.1055/s-0037-1602697.
- Prabhakar H, Mahajan C, Kapoor I. Anesthesia for minimally-invasive neurosurgery. Curr Opin Anaesthesiol. 2017;30(5):546-550. doi:10.1097/ACO.0000000000000499.
- Pulhorn H, Quigley DG, Bosma JJ, et al. Impact of brain biopsy on the management of patients with nonneoplastic undiagnosed neurological disorders. Neurosurgery. 2008;62(4):833-837. doi:10.1227/01.neu.0000318168.97966.17.
- Ringel F, Ingerl D, Ott S, Meyer B. VarioGuide: a new frameless image-guided stereotactic system–accuracy study and clinical assessment. Neurosurgery. 2009;64(5 Suppl 2):365-371. doi:10.1227/01.NEU.0000341532.15867.1C.
- Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315-e329. doi:10.1016/S1470-2045(17)30194-8.
- Nakagawa JM, Trippel M, Doostkam S, Mader I, Coenen VA, Reinacher PC. The stereotactic suboccipitaltranscerebellar approach to lesions of the brainstem and the cerebellum. Clin Neurol Neurosurg. 2018;166:10-15. doi:10.1016/j.clineuro.2018.01.015.