Prophylactic Laparoscopic Bilateral Gonadectomy for Complete Androgen Insensitivity Syndrome
Abstract
Androgen insensitivity syndrome (AIS) is a rare condition caused by an X-linked mutation of the androgen receptor with an estimated incidence of 1–5 per 100,000 individuals. Varying degrees of presentation exist for complete, partial, or mild depending on the severity of androgen resistance. Patients with complete AIS (CAIS) are born phenotypically female but have male XY chromosomes and testes instead of ovaries. They exhibit normal secondary female sex characteristics such as breast development and external female genitalia but lack a uterus and other Müllerian duct structures due to testicular production of Müllerian-inhibiting factor (MIF). Due to androgen-resistance, androgen-dependent Wolffian duct products fail to develop such as the epididymis, vas deferens, and the seminal vesicles. These patients often present either during infancy with inguinal hernias or sublabial masses, or during adolescence with primary amenorrhea. On physical exam, they will typically have normal breast development, lack pubic or axillary hair, and will have a blind-ending vaginal pouch of varying vaginal lengths. Diagnostic work-up is often conducted using ultrasound or MRI, serum hormone levels, and karyotype analysis.
For patients with CAIS, their testes can be located within the inguinal canal, sublabially or intra-abdominally. Following puberty, patients with intra-abdominal testes are at a 15% increased risk (range 0–22%) of developing germ cell tumors (GCT). Management consists of prophylactic gonadectomy with subsequent hormone replacement therapy (HRT) to maintain normal pubertal development and promote adequate bone health. The debate regarding the timing of prophylactic gonadectomy is ongoing with some patient support groups arguing against gonadectomy citing concerns with long-term hormone therapy and the desire to preserve fertility. Current convention promotes delaying gonadectomy until after physiologic puberty has been achieved as the risk of developing prepubertal GCT is relatively low (0.8–2%). We outline the presentation, diagnosis, intraoperative techniques, and postoperative considerations for managing CAIS via bilateral laparoscopic gonadectomy.
Case Overview
Background
Gonadectomy may be indicated in children with Differences of Sexual Development (DSD) who harbor Y-chromosome gonads due to the increased risk of gonadal malignancy.1 One such DSD is androgen insensitivity syndrome (AIS), which is caused by an X-linked mutation of the androgen receptor (AR).2 AIS is a rare diagnosis with an estimated incidence between 1–5 per 100,000 individuals.3 AIS can have varying degrees of presentation depending on the severity of androgen resistance, ranging from complete (CAIS), partial (PAIS), and mild (MAIS).4 Children with CAIS are phenotypically female in appearance with normal female external genitalia but have testes instead of ovaries and have a male karyotype (46, XY). In these patients, their testes are able to produce testosterone but, due to the defect in AR function, fail to produce Wolffian duct products such as the epididymis, vas deferens, and the seminal vesicles. Due to peripheral aromatization of this testosterone into estrogen, these patients have normal secondary female sex characteristics such as breast development. Nevertheless, the Sertoli cells of the testes continue to produce Müllerian-inhibiting factor (MIF), which inhibits the development of the Müllerian duct derivatives. This results in patients with a blind-ending vaginal pouch with an absence of other female sex organs such as the uterus, cervix, and fallopian tubes.5 In these patients, the testes can be located within the inguinal canal, be sublabial, or be intra-abdominal.6 Infants with androgen insensitivity may present with unilateral or bilateral inguinal hernias or labial masses. It is estimated that 1–2% of bilateral inguinal hernias among girls could represent a CAIS diagnosis, and it is important to maintain a strong clinical suspicion during your evaluation.7 Classically, CAIS presents during adolescence as primary amenorrhea in girls with normal breast development but little to no pubic or axillary hair on examination. CAIS is associated with abnormal testicular development as well as an increased risk of germ cell malignancy following puberty.8
Focused History of the Patient
This patient is a 15-year-old female of Asian descent with a past medical history of morbid obesity (BMI of 45), obstructive sleep apnea on CPAP, and prediabetes (HbA1c of 5.5) currently on metformin. She has a past surgical history of bilateral inguinal hernia repair as a young child. She has no history of previous pregnancies, is not sexually active, and was referred to our clinic for further evaluation of primary amenorrhea. She reports that she developed thelarche at age 11, but has sparse axillary and pubic hair and no signs of acne. In addition, she denies any pain, vaginal discharge, hirsutism, or galactorrhea. At an outside clinic, our patient denied bleeding following a progestin challenge, and no cervix was palpated during a previous pelvic exam.
Per the patient’s family history, she has three older sisters who are 19, 21, and 26 years of age. Her mother started menses at age 14. Her 26-year-old and 19-year-old sisters also report having regular menses that started around the age of 11, and the oldest sister has had four healthy children. Interestingly, her 21-year-old sister has also been seen by an out-of-state physician for evaluation of primary amenorrhea of unknown etiology. She was told that her vaginal hiatus was insufficient for childbirth and was referred to a specialist for further evaluation but was lost to follow-up.
Physical Exam
On physical exam, the patient was obese, had Tanner stage 5 breast development with sparse axillary hair and no signs of acne present. On genitourinary exam, the patient had normal external female genitalia with sparse pubic hair. The vagina was normal in appearance, with no abnormal discharge, and a blind-ending vaginal pouch. We were unable to visualize or palpate the cervix or uterus on the exam. Exam findings were consistent for a potential congenital abnormality. Appropriate lab and imaging tests were then ordered.
Lab Results:
| Test | Result | Reference |
| B-hCG | Negative | Negative |
| FSH | 13.20 mIU/mL | 5–20 mIU/mL |
| LH | 22.20 mIU/mL | 5–20 mIU/mL |
| Prolactin | 8.1 ng/mL | 3.0–18.6 ng/mL |
| Estradiol | 38.33 pg/mL |
34–170 pg/mL (Female Tanner Stage 5) |
| Testosterone | 269 ng/dL |
20–38 ng/dL (Female Tanner Stage 5) |
| TSH | 1.94 mIU/L | 0.47–4.68 mIU/L |
| Chromosome Analysis | 46, XY |
Female: 46, XX Male: 46, XY |
Initial labs were drawn to assess problems associated with the hypothalamic-pituitary-ovarian (HPO) axis, such as follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin, and testosterone. In addition, a urine beta-hCG was conducted to rule out pregnancy. Labs for our patient revealed that she had an elevated testosterone level when compared to normal female patients. In addition, a karyotype analysis for our patient resulted in 46, XY chromosomes. Together, these findings are consistent with a diagnosis of CAIS, where elevated testosterone levels (abnormal female range, but normal male range) and high serum LH levels result due to impairment of androgen negative feedback on the anterior pituitary.
Imaging
Imaging via pelvic ultrasound was ordered to assess for the presence or absence of the uterus and other female sex organs. Ultrasound imaging conducted at an outside hospital showed an absence of a uterus, fallopian tubes, and cervix. In addition, imaging revealed an absence of ovaries and was unable to accurately assess the location of potential intra-abdominal testes. Ultrasounds can be operator dependent, and MRI is widely considered to be the gold standard to diagnose and locate the gonads in surgical planning for laparoscopic gonadectomy and gonadal surveillance.5
Natural History
One study by Wisniewski et al. examined long-term outcomes among 14 women with CAIS who were on long-term hormone replacement therapy (HRT) following gonadectomy.9 They found that overall these women can expect to have a normal active life span. A majority of these women exceeded the 90th percentile for height for normal adult females,9 making them taller than the average female while still being shorter than the normal male population.10 The most common medical condition that was diagnosed among these women was osteoporosis. The majority of women identified as having a heterosexual female gender identity and none of them desired gender reversal surgery. The majority reported being satisfied with their sexual functioning. The average vaginal length among this cohort was 8.8 cm, which is consistent with normal vaginal length ranging from 7–11 cm.9
Options for Treatment
Currently, there is no therapy available to reverse the underlying genetic mutation of the AR in patients with CAIS. Therefore, treatment is focused on prophylactic gonadectomy to prevent potential gonadal malignancy with subsequent HRT, treatment of the urogenital tract, if indicated, as well as psychological support. Gonadectomy is usually delayed until sexual maturation is complete during adolescence to allow for normal spontaneous pubertal development.3 If diagnosed early in infancy or childhood, early gonadectomy may be considered if the child presents with painful or uncomfortable inguinal or labial masses, but will require subsequent HRT to induce puberty at approximately 11–12 years of age.3 The timing of gonadectomy has become controversial with some patients and AIS support groups advocating for retaining their testes. A variety of reasons have been cited by these support groups for keeping the testes such as psychological factors, risks associated with the surgery, desire to potentially preserve fertility, and a reluctance to adhere to long-term HRT. Nevertheless, the reported risks for laparoscopic gonadectomy are very low, with an estimated risk of death of 0.1 per 1000 procedures, and the risk of injury to bowel or bleeding is reported at 2.4%.11 Furthermore, a study by Hannema et al. examined the testes of 44 patients with CAIS and found that germ cells rapidly declined in number after the first year of life and found no evidence of spermatogenesis in any of the testes, making fertility highly unlikely for CAIS patients.12 For CAIS patients who decide to retain their testes, it has been reported that the risk of developing germ cell tumors (GCT) increases with age.11 It is important to maintain close follow-up through active surveillance by utilizing regular imaging (ultrasound and/or MRI) and serum blood markers to screen for the potential development of GCT in these patients.13 While MRI can detect benign changes such as paratesticular cysts and adenomas, they cannot detect premalignant changes such as germ cell neoplasia in situ (GCNIS), which would require a biopsy of the gonads.14 In addition, the quality of ultrasound screening is usually operator dependent. One approach proposed by Wunsch et al. for patients who desire to retain their testes, would be to perform laparoscopic gonadal biopsy and surgically fix the intra-abdominal gonads near the abdominal wall to allow for better visualization via ultrasound.15
Rationale for Treatment
The primary goal of performing gonadectomy in the setting of CAIS is to lessen the risk of future malignancy. As in other forms of cryptorchidism, there is an increased risk of developing GCT. In CAIS, the risk of developing prepubertal GCT among these patients is considered to be very low ranging from 0.8–2.0%.16 Following puberty, this risk increases with age and is estimated to be roughly 15% (ranges from 0–22%).11 It is advocated that prophylactic gonadectomy occurs in the postpubertal period when feminization is completed in part by testicular estrogen that is partly derived from the conversion of androgens to estrogen.3, 8 Delaying gonadectomy until later in adolescence also allows care providers to obtain informed consent directly from their patients.
Special Considerations
When evaluating children, whose HPO axis is still immature, an hCG stimulation test is necessary to properly evaluate Leydig cell testosterone secretion.17 Following gonadectomy, these patients will need long-term hormonal supplement therapy with estrogen replacement until the age of natural menopause (around 50–52 years of age) to maintain normal breast and bone development, psychosocial well-being, and sexual function.18 Because these patients do not have a uterus, progestins are not required to complement estrogen therapy.17 These patients will continue to retain normal secondary female sex characteristics and can attain normal sexual function but may require vaginal dilation therapy or vaginoplasty depending on the adequacy of their vaginal canal.17 Questions of infertility and gender identity can carry a heavy psychosocial impact for these patients, and it is strongly encouraged to offer counseling or support group therapy as part of a multi-disciplinary approach.9
Discussion
CAIS (formerly known as Morris syndrome) represents one of the most common definable causes of 46, XY DSD. It results from a rare X-linked mutation of the AR that causes peripheral androgen resistance. These patients are born phenotypically female with normal female external genitalia. Commonly, these patients present in adolescence with primary amenorrhea, where the subsequent examination will reveal that these patients have a blind-ending vaginal pouch and an absence of internal female sex organs on imaging. Instead of ovaries, these patients have testes that may be found in the abdomen, inguinal canal, or labia. In children or infants, CAIS may present as an inguinal hernia or mass, where approximately 1–2% of female infants with inguinal hernias are found to have cryptorchidism with 46,XY karyotype.7 In this specific case, our patient had a past surgical history of bilateral inguinal hernia repair as a young child. This suggests a missed or delayed diagnosis and highlights the importance of maintaining a strong clinical suspicion of CAIS in pediatric female patients who present with bilateral inguinal hernias, which warrants further examination to rule out cryptorchidism.
The estimated risk for patients with AIS developing GCT is inversely related to the degree of androgen resistance. Patients with CAIS have more severe mutations of their AR that infer a complete loss of function. Without androgen stimulation, spermatogenesis is impaired and there is an associated rapid decline in germ cell numbers after the first year of life that theoretically confers a reduced risk of developing GCT later in life.14 This is in contrast to patients with PAIS, who still retain some degree of AR function and therefore are more likely to have surviving germ cells, which subsequently puts them at increased risk for developing GCT in adulthood.19 Historically, Manuel et al. reported a 3.6% cumulative risk of GCT in patients with Y-containing DSDs up to the age of 25 years that increased to 33% by 50 years of age.20 More recently, Deans et al. found in their review that CAIS patients were at a 15% increased risk of developing a gonadal malignancy in adulthood (range 0–22%).11 Cools et al. found that the estimated risk of developing GCT in CAIS patients before puberty was much lower at 0.8–2%.16
Due to the increased risk of gonadal malignancy among CAIS patients in adulthood, the current recommendation is to perform gonadectomy after sexual maturation is complete, typically around 15–16 years of age, as the risk of developing tumors before puberty is considered to be relatively low.21 This approach allows for spontaneous breast development and better bone mineralization during puberty due to physiologic hormone production by the testes and subsequent peripheral androgen conversion to estrogens.3, 8 Historically, laparotomy and bilateral gonadectomy were performed for patients with Y-chromosome containing DSD. Overtime, laparoscopic procedures became widely adopted for DSD patients due to the associated advantages of magnification and easy access to the pelvic cavity via a minimally invasive approach, which provides shorter postoperative recovery and length of hospitalization, and improved cosmesis.22, 23
Laparoscopic gonadectomy is performed while the patient is under general anesthetic via endotracheal intubation. The video monitor, insufflator, and light source are positioned at the foot of the patient. In this case, insufflation of the abdomen was performed using an open laparoscopy technique where a semilunar incision was made at the inferior aspect of the umbilicus and the fascia was elevated using hemostats. A Veress needle was then placed into the abdomen, and its correct position was confirmed using a saline drop test. A 10-mm Step trocar was then inserted through the umbilicus, and CO2 was used to obtain pneumoperitoneum. A 0o laparoscope was then introduced into the abdomen. Two additional 5-mm trocars for working instruments were placed at the level of the umbilicus on the right and left sides. The patient was then placed into Trendelenburg position, which allows for easier laparoscopic inspection of the pelvis to determine the location of the gonads and to inspect the pelvic organs. When the gonads are not readily apparent, identifying and following the gonadal vessels can help locate them.1
During laparoscopy, our patient’s gonads were noted above the closed internal rings bilaterally. Grossly, cysts were visualized on bilateral testes. The vas deferens traversed to the urethra, and there was no evidence of any Müllerian structures within the pelvis. A plane was dissected through the posterior peritoneum around the gonads away from the other retroperitoneal structures. It is important to determine the location and course of retroperitoneal structures such as the ureters and the iliac vessels to avoid iatrogenic injury. The internal spermatic vessels were then identified as they transverse to the gonad and fulgurated using the Ligasure device in four successive sections before transecting to reduce the likelihood of bleeding. The testes were then mobilized from the peritoneum, and the vas deferens were also fulgurated and divided in a similar fashion. The laparoscope was inserted through one of the working ports so that the gonads could be removed through the central 10-mm umbilical port. The abdominal CO2 pneumoperitoneum was then reversed, and the umbilical fascia was closed using 2-0 Vicryl suture. The skin at all of the port sites was closed using 5-0 Monocryl and covered with Dermabond.
Both of the gonads were excised without complication and sent to pathology for evaluation. The laparoscopic gonadectomy operating time, from incision to closure, was approximately 80 minutes. There was less than 5 mL of estimated blood loss. The patient was admitted overnight for observation due to social factors. She tolerated the procedure well, her pain was well controlled using multi-modal pain management, and she was discharged home the following morning. She was scheduled for follow-up in two weeks with her gynecologist to begin estrogen replacement therapy.
It is significant to note that under laparoscopy we were able to visualize bilateral vas deferens that traced down to the urethra. A case series of 44 CAIS patients by Hannema et al. found that 36% had epididymis or vas deferens present.12 Hannema et al. hypothesized that residual paracrine androgen activity may be able to induce the development of Wolffian duct products, even in patients with complete forms of AIS.12
Surgical pathology for our patient confirmed that both of her gonads were in fact atrophic testes. Interestingly, both testes demonstrated GCNIS and marked Leydig cell hyperplasia (Figures 1–2). The neoplastic cells stain with OCT3/4 and PLAP (Figures 3–4). Leydig cell hyperplasia, as seen in this patient, is a common finding in patients with CAIS.24 It has been proposed that high levels of LH, due to a lack of androgen negative feedback on the anterior pituitary, is responsible for increased Leydig cellularity.25 GCNIS is considered to be a premalignant tumor, where up to 50% will progress to GCT within 5 years.26 The risk of progression of GCNIS to invasive GCT is less certain in patients with CAIS. The “lack-of-androgen theory” proposed by Kaprova-Pleskacova et al., suggests that patients with CAIS are less likely to progress to GCT than patients with PAIS due to insufficient androgen response to promote survival of abnormal germ cells.27 Conversely, Kaprova-Pleskacova et al. also suggested that the same paracrine androgen activity potentially responsible for inducing Wolffian duct development as mentioned by Hannema et al.,12 could also promote GCNIS to develop into invasive GCT.27 The presence of vas deferens and histological evidence of GCNIS in our patient suggests potential residual paracrine androgen response. As such, we believe this case helps support the argument for the potential benefit of prophylactic gonadectomy among CAIS patients.
Our patient’s surgical pathology was also significant for bilateral paratesticular leiomyomas (Figure 5), a smooth muscle tumor that very rarely occurs within the urogenital tract. Their location may be intratesticular or paratesticular and are believed to be derived from the smooth muscle cells of the interstitial stroma, the muscular layer of the vessels of the tunica albuginea, the seminiferous tubules, as well as the paratesticular structures such as the spermatic cord, epididymis, vestigial remnants, and the tunica vaginalis.28 Leiomyomas are very rarely described in patients with AIS. In fact, there have only been four case reports within the literature describing leiomyomas being present on biopsy following gonadectomy in AIS patients.28-31 To our knowledge, this is the first documented case of bilateral paratesticular leiomyomas developing concurrently with GCNIS in a patient with CAIS.
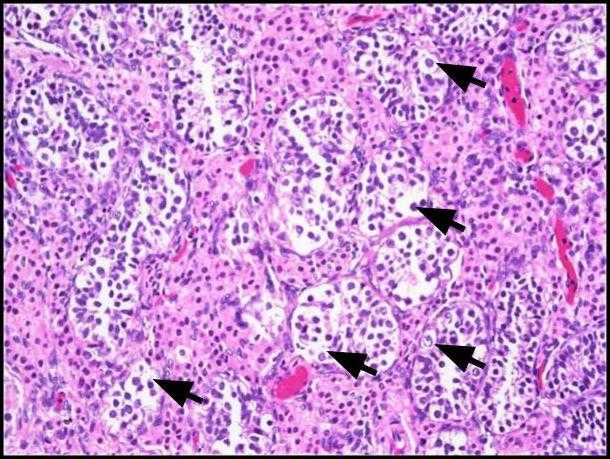
Figure 1. Germ cell neoplasia in situ (GCNIS). Large atypical cells (black arrows) with abundant clear to faintly eosinophilic cytoplasm, a central nucleus with the prominent nucleolus, and evenly distributed chromatin are seen. H&E, 200x.
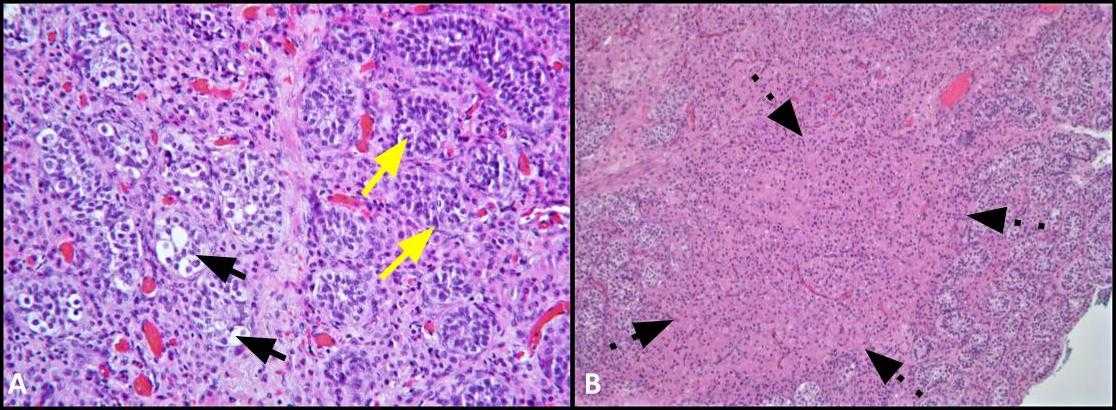
Figure 2. A) This image shows comparison of tubules involved by GCNIS (black arrows) with adjacent seminiferous tubules with normal spermatogenesis (yellow arrows); H&E, 200x. B) Low power image shows clusters of Leydig cells within the interstitium (dashed arrows); H&E, 100x.
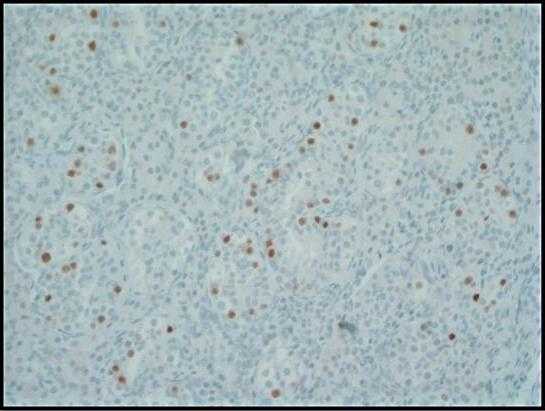
Figure 3. Immunohistochemical stain for octamer-binding transcription factor (OCT) 3/4 shows strong nuclear immunoreactivity in GCNIS cells within the seminiferous tubules. Normal germ cells are negative; 200x.
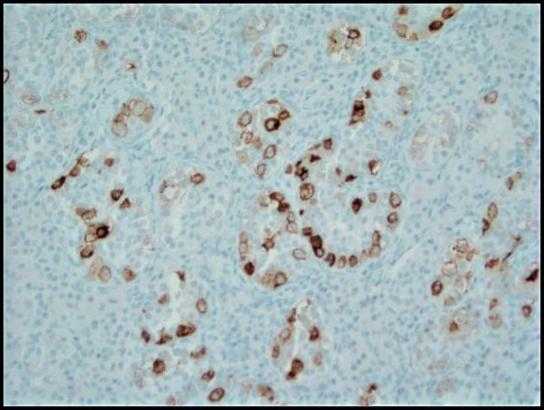
Figure 4. Immunohistochemical stain with placental alkaline phosphatase (PLAP) highlights the tumor cells of GCNIS in a cytoplasmic membranous pattern; 200x.
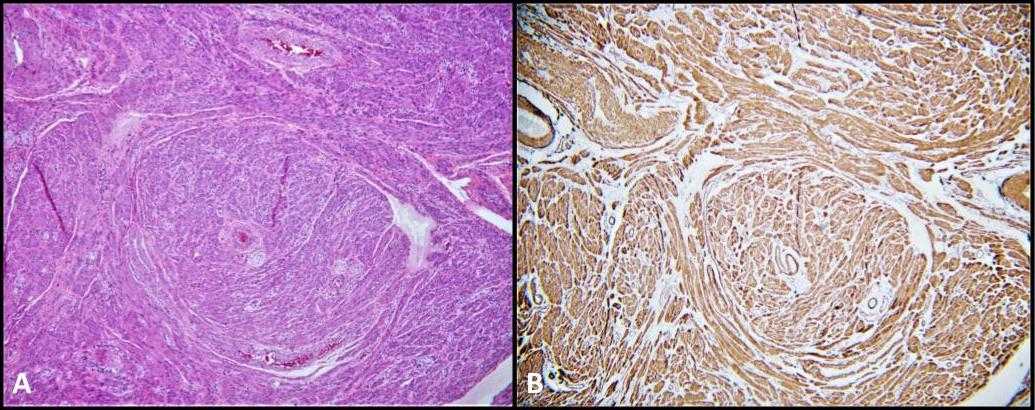
Figure 5. A) Leiomyoma; H&E, 100x. Fascicles of spindled smooth muscle cells. B) Smooth muscle actin (SMA) cytoplasmic staining is diffuse and strong.
Equipment
No specific equipment used.
Disclosures
Nothing to disclose.
Statement of Consent
The patient referred to in this video article has given their informed consent to be filmed and is aware that information and images will be published online.
Citations
- Calvo A, Escolino M, Settimi A, Roberti A, Caprio MG, Esposito C. Laparoscopic approach for gonadectomy in pediatric patients with intersex disorders. Transl Pediatr. 2016;5(4):295-304. https://doi.org/10.21037/tp.2016.09.06
- Kusumi M, Mitsunami M, Onoue H, et al. Complete androgen insensitivity syndrome and anti-Müllerian hormone levels before and after laparoscopic gonadectomy. Gynecol Minim Invasive Ther. 2017;6(3):126-128. https://doi.org/10.1016/j.gmit.2016.11.001
- Cheikhelard A, Thibaud E, Morel Y, et al. Complete androgen insensitivity syndrome: diagnosis and management. Expert Rev Endocrinol Metab. 2009;4(6):565-573. https://doi.org/10.1586/eem.09.31
- Lanciotti L, Cofini M, Leonardi A, Bertozzi M, Penta L, Esposito S. Different Clinical Presentations and Management in Complete Androgen Insensitivity Syndrome (CAIS). Int J Environ Res Public Health. 2019;16(7):1268. https://doi.org/10.3390/ijerph16071268
- Grasso D, Borreggine C, Campanale C, Longo A, Grilli G, Macarini L. Usefulness and role of magnetic resonance imaging in a case of complete androgen insensitivity syndrome. Radiol Case Rep. 2015;10(2):1119. https://doi.org/10.2484/rcr.v10i2.1119
- Nezzo M, De Visschere P, T'Sjoen G, Weyers S, Villeirs G. Role of imaging in the diagnosis and management of complete androgen insensitivity syndrome in adults. Case Rep Radiol. 2013;2013:158484. https://doi.org/10.1155/2013/158484
- Viner RM, Teoh Y, Williams DM, Patterson MN, Hughes IA. Androgen insensitivity syndrome: a survey of diagnostic procedures and management in the UK. Arch Dis Child. 1997;77(4):305-309. https://doi.org/10.1136/adc.77.4.305
- Galani A, Kitsiou-Tzeli S, Sofokleous C, Kanavakis E, Kalpini-Mavrou A. Androgen insensitivity syndrome: clinical features and molecular defects. Hormones (Athens). 2008;7(3):217-229. https://doi.org/10.14310/horm.2002.1201
- Wisniewski AB, Migeon CJ, Meyer-Bahlburg HF, et al. Complete androgen insensitivity syndrome: long-term medical, surgical, and psychosexual outcome. J Clin Endocrinol Metab. 2000;85(8):2664-2669. https://doi.org/10.1210/jcem.85.8.6742
- Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K, MacDougall J. Androgen insensitivity syndrome. Lancet. 2012;380(9851):1419-1428. https://doi.org/10.1016/S0140-6736(12)60071-3
- Deans R, Creighton SM, Liao LM, Conway GS. Timing of gonadectomy in adult women with complete androgen insensitivity syndrome (CAIS): patient preferences and clinical evidence. Clin Endocrinol (Oxf). 2012;76(6):894-898. https://doi.org/10.1111/j.1365-2265.2012.04330.x
- Hannema SE, Scott IS, Rajpert-De Meyts E, Skakkebaek NE, Coleman N, Hughes IA. Testicular development in the complete androgen insensitivity syndrome. J Pathol. 2006;208(4):518-527. https://doi.org/10.1002/path.1890
- Döhnert U, Wünsch L, Hiort O. Gonadectomy in Complete Androgen Insensitivity Syndrome: Why and When? Sex Dev. 2017;11(4):171-174. https://doi.org/10.1159/000478082
- Chaudhry S, Tadokoro-Cuccaro R, Hannema SE, Acerini CL, Hughes IA. Frequency of gonadal tumours in complete androgen insensitivity syndrome (CAIS): A retrospective case-series analysis. J Pediatr Urol. 2017;13(5):498.e491-498.e496. https://doi.org/10.1016/j.jpurol.2017.02.013
- Wünsch L, Holterhus PM, Wessel L, Hiort O. Patients with disorders of sex development (DSD) at risk of gonadal tumour development: management based on laparoscopic biopsy and molecular diagnosis. BJU Int. 2012;110(11 Pt C):E958-965. https://doi.org/10.1111/j.1464-410X.2012.11181.x
- Cools M, Drop SL, Wolffenbuttel KP, Oosterhuis JW, Looijenga LH. Germ cell tumors in the intersex gonad: old paths, new directions, moving frontiers. Endocr Rev. 2006;27(5):468-484. https://doi.org/10.1210/er.2006-0005
- Batista RL, Costa EMF, Rodrigues AdS, et al. Androgen insensitivity syndrome: a review. Archives of Endocrinology and Metabolism. 2018;62:227-235. https://doi.org/10.20945/2359-3997000000031
- Bertelloni S, Meriggiola MC, Dati E, Balsamo A, Baroncelli GI. Bone Mineral Density in Women Living with Complete Androgen Insensitivity Syndrome and Intact Testes or Removed Gonads. Sex Dev. 2017;11(4):182-189. https://doi.org/10.1159/000477599
- Pyle LC, Nathanson KL. A practical guide for evaluating gonadal germ cell tumor predisposition in differences of sex development. Am J Med Genet C Semin Med Genet. 2017;175(2):304-314. https://doi.org/10.1002/ajmg.c.31562
- Manuel M, Katayama PK, Jones HW, Jr. The age of occurrence of gonadal tumors in intersex patients with a Y chromosome. Am J Obstet Gynecol. 1976;124(3):293-300.
https://doi.org/10.1016/0002-9378(76)90160-5 - Chertin B, Koulikov D, Alberton J, Hadas-Halpern I, Reissman P, Farkas A. The use of laparoscopy in intersex patients. Pediatr Surg Int. 2006;22(5):405-408. https://doi.org/10.1007/s00383-006-1662-3
- Esegbona G, Cutner A, Cuckow P, Creighton S. Laparoscopic gonadectomy in paediatric and adolescent girls with intersex disorders. BJOG: An International Journal of Obstetrics & Gynaecology. 2003;110(2):210-212. PMID: 12618168.
- Cools M, Looijenga L. Update on the Pathophysiology and Risk Factors for the Development of Malignant Testicular Germ Cell Tumors in Complete Androgen Insensitivity Syndrome. Sex Dev. 2017;11(4):175-181. https://doi.org/10.1159/000477921
- Jockenhövel, Rutgers JK, Mason JS, Griffin JE, Swerdloff RS. Leydig cell neoplasia in a patient with Reifenstein syndrome. Exp Clin Endocrinol. 1993;101(6):365-370.
- Akyüz M, Topaktaş R, Ürkmez A, Koca O, Öztürk M. Evaluation of germ-cell neoplasia in situ entity in testicular tumors. Turk J Urol. 2019;45(6):418-422. https://doi.org/10.5152/tud.2018.48855
- Kaprova-Pleskacova J, Stoop H, Brüggenwirth H, et al. Complete androgen insensitivity syndrome: factors influencing gonadal histology including germ cell pathology. Modern Pathology. 2014;27(5):721-730. https://doi.org/10.1038/modpathol.2013.193
- Siminas S, Kokai G, Kenny SE. Complete androgen insensitivity syndrome associated with bilateral Sertoli cell adenomas and paratesticular leiomyomas: case report and review of the literature. J Pediatr Urol. 2013;9(1):e31-34. https://doi.org/10.1016/j.jpurol.2012.06.013
- Goulis DG, Iliadou PK, Papanicolaou A, et al. R831X mutation of the androgen receptor gene in an adolescent with complete androgen insensitivity syndrome and bilateral testicular hamartomas. Hormones (Athens). 2006;5(3):200-204. https://doi.org/10.14310/horm.2002.11185
- Krichen Makni S, Mnif Hachicha L, Ellouze S, et al. Feminizing testicular syndrome with multiple hamartomas and bilateral paratesticular leiomyomas. Rev Med Interne. 2005;26(12):980-983. https://doi.org/10.1016/j.revmed.2005.08.003
- Savaş-Erdeve Ş, Aycan Z, Keskin M, et al. Complete androgen insensitivity syndrome associated with bilateral sertoli cell adenomas and unilateral paratesticular leiomyoma: A case report. The Turkish Journal of Pediatrics. 2016;58(6):654-657. https://doi.org/10.24953/turkjped.2016.06.012
