Thoracofemoral Bypass: A Retroperitoneal Approach
Abstract
Surgical intervention for aortoiliac occlusive disease (AIOD) remains a vital tool in the management of AIOD. AIOD is caused by occlusion of the infrarenal and/or iliac arteries, often secondary to atherosclerosis. Here, we present a case of a young, male patient with a history of familial hyperlipidemia and chronic tobacco use who underwent a thoracofemoral bypass (TFB) procedure via a retroperitoneal approach. He presented with classic symptoms of bilateral leg pain when walking, nocturnal lower extremity pain, and correlated diminished lower extremity pulses. TFB was the preferred approach due to the aggressive, soft plaque burden extending into the suprarenal aorta, which precluded endovascular repair and would have increased risk for standard infrarenal aortofemoral bypass (AFB). This video and case report present a detailed explanation of a retroperitoneal approach to a TFB procedure and the nuanced indications of the surgical interventions for AIOD.1,2
Case Overview
Background
Aortoiliac occlusive disease (AIOD) is a complex manifestation of peripheral arterial disease (PAD), in which the lumen of the infrarenal aorta and/or the iliac arteries are obliterated secondarily to atherosclerosis (often a mix of calcified and lipid-rich plaque). Symptomatic PAD can be caused by primary atherosclerotic plaques narrowing the lumen and limiting flow, or by secondary obstruction from the embolic complications of these plaques.1,2 AIOD frequently presents with symptoms that progress from buttock/thigh claudication, to rest pain in the lower extremities, and, in the most severe form, to ischemic ulceration. The classic description for the presentation of AIOD was made by Leriche, with his eponymous syndrome consisting of buttock/thigh claudication, absent femoral pulses, and erectile dysfunction.1,2 When presenting with concomitant distal embolization or infrainguinal occlusion, AIOD can result in chronic limb-threatening ischemia (CLTI), which has a poor prognosis.3
The most significant risk factors for atherosclerosis, and consequently AIOD, are tobacco usage and diabetes mellitus.1 Hyperlipidemia, which can be lifestyle-induced or due to early-onset familial hyperlipidemia, also contributes to the development of AIOD. Other risk factors include increased age, familial history, male sex, and race.4,5
The prevalence of AIOD in the general population ranges from 3.56% to greater than 14%.4,6 Studies have shown a higher prevalence in the 70 to 80 age range of 14% to 23%.8,9 As the population continues to age and rates of diabetes and cardiovascular disease rise, there will be the potential for a greater burden of AIOD. Thus, it is important to identify uniquely at-risk patients to obtain early intervention.
Work-up should be initiated with an Ankle-Brachial Index (ABI).10-12 Clinical suspicion for AIOD should be high with an abnormal ABI and absent/abnormal femoral pulse exam. In the symptomatic patient, the work-up should proceed with a computed tomography arteriogram (CTA) to delineate the nature of the disease and make appropriate risk stratification for intervention. However, there still are some patients that are symptomatic and can be managed initially by conservative measures like walking programs and cilostazol. These patients do not need to undergo CTA as it would be unwanted radiation and contrast medium exposure.13,14
Regardless of intervention strategy, all patients need optimal medical management of chronic diseases, including evaluation for statin and antiplatelet administration, employing an exercise regimen, and smoking cessation.11,15,16 For those in whom intervention is warranted, endovascular therapy is often the first line of treatment, with surgical bypass reserved for those with either concomitant aneurysm or more extensive disease burden.
Focused History of the Patient
The patient is a 52-year-old gentleman with a past medical history of early-onset familial hyperlipidemia and past tobacco use (50 pack-year) who initially presented for coronary artery bypass grafting (CABG) secondary to an acute myocardial infarction. During the clinical evaluation for the CABG, he was found to have an infrarenal aortic occlusion. After undergoing a successful CABG and cardiac rehabilitation, he was evaluated in a vascular clinic. The patient reported success with smoking cessation after the CABG, but still endorses short-distance claudication with cramping pain in the bilateral thighs/buttocks/calves at 50 yards. Furthermore, he had a significant personal history of hyperlipidemia and a family history of several first-degree relatives with index cardiovascular events under the age of 50. He appeared healthy without ischemic wounds but had absent femoral/popliteal and pedal pulses.
Imaging
CTA demonstrated a complete aortic occlusion at the level of the bilateral renal arteries from a combination of calcified and soft atherosclerotic plaque. Significantly, in this case, the atheroma extended proximally to the level of the superior mesenteric artery (SMA), as seen in Figure 1, which would have complicated suprarenal clamping and compromised the appropriate inflow for standard aortofemoral bypass (AFB). The supraceliac aorta was free of arterial disease (Figure 2). The occlusion extended to the bilateral common femoral arteries, where flow was reconstituted from the epigastric and circumflex iliac arterial collaterals. The bilateral common femoral arteries (CFA) had atheromatous plaque of approximately 60% stenosis, and then the runoff was intact below the CFA bifurcation.
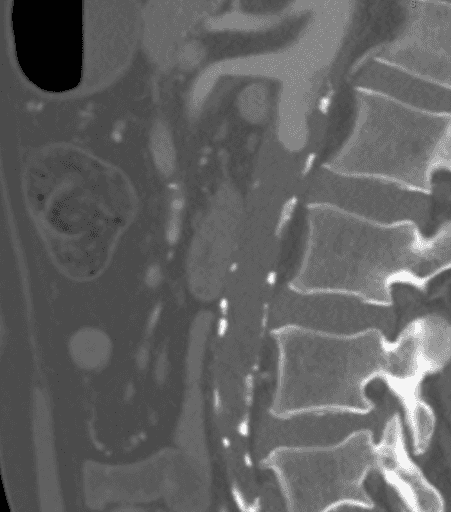
Figure 1. CTA showing that the atheroma extended proximally to the level of the superior mesenteric artery (SMA), which would have complicated suprarenal clamping and compromised the appropriate inflow for standard AFB.
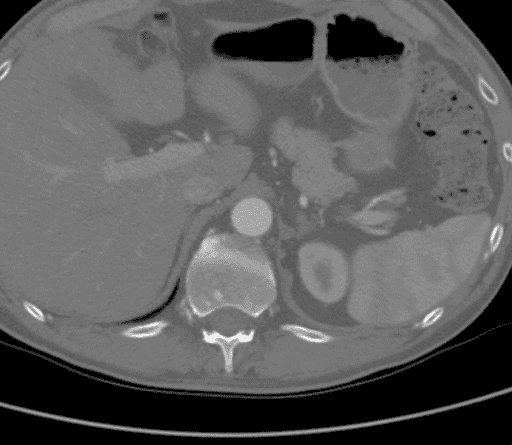
Figure 2. CTA showing that the supraceliac aorta was free of arterial disease.
Options for Treatment
As with any patient with claudication, the first option for treatment is conservative, with optimal medical therapy and smoking cessation. However, the durability of inflow procedures, combined with low morbidity and mortality in appropriately selected patients, makes intervention appropriate for patients such as those who remain symptomatic despite conservative measures. The Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II)17 is an excellent guide to describe the various treatment options and the rationale for the appropriate choice based on patient anatomy and comorbidities. In general, for patients such as this one, with flush aortic occlusion and external iliac occlusion that will require CFA endarterectomy, an open surgical approach is the preferred management. Open surgical bypass from the infrarenal aorta to the bilateral CFA +/- CFA endarterectomy is the most common reconstruction for severe AOID, with 10-year patency reported as high as 90%.17 However, certain anatomic constraints such as the burden of disease in the proximal clamp site or failed prior AFB may limit the standard approach, in which case a bypass to the bilateral CFA using the descending thoracic aorta as inflow is a viable approach. Patency at 5 years is greater than 80% when done via either a thoracotomy or retroperitoneal approach to the distal descending thoracic aorta.18 In a patient with severe medical comorbidities precluding aortic level operation, axillo-bifemoral bypass with concomitant CFA endarterectomy would be another viable option, although with an expected patency of only ~50% at 5 years.
Rationale for Treatment
This patient is young and fit after smoking cessation and successful coronary revascularization. He remained symptomatic and, given his age, still desired improved walking distance to facilitate employment, daily lifestyle, and improved exercise capacity for better long-term health.
The decision to use an alternate aortic inflow site at the descending thoracic aorta was mostly made secondary to the soft atheroma within a typical clamp site juxtarenal, which would have compromised the standard proximal aortic anastomosis. Furthermore, the decision was made to use a retroperitoneal approach as opposed to a left thoracotomy as this would allow for intraoperative duplex evaluation of the visceral and renal vessels to ensure adequate inflow and perform concomitant visceral bypass if needed. Additionally, given his youth, we could leave the thoracic cavity inflow site for future revascularization if needed.
Discussion
Operative Sequence
The steps of a thoracofemoral bypass (TFB) should be familiar to most vascular surgeons as they mimic many of the same sequencing as a standard AFB, just with an alternate inflow site. The patient is positioned in a modified right lateral decubitus with the hips as close to flat as possible to allow for adequate femoral access to perform endarterectomy when indicated, such as this patient. The operation should begin with the groin exposures through oblique incisions to limit the overall time the retroperitoneum (RP) is open to decrease insensible losses. When concomitant femoral endarterectomy is anticipated, it is crucial to control the superficial femoral artery (SFA) and profunda femoris (PA) down to zones free of plaque to ensure adequate endarterectomy. The main site of failure in AFB is the femoral anastomoses, thus, meticulous attention is warranted to this step.
After adequate arterial dissection, the inguinal ligament should be divided slightly to avoid compression of the graft limbs as they pass into the femoral region. This also allows for direct visualization of the superficial circumflex iliac vein as it courses over the distal external iliac artery (EIA) and ligation prior to tunneling. The groins should be packed with moist gauze and attention turned to the aortic exposure.
A curvilinear incision is then made starting at the 8th or 9th intercostal space in most patients as this will facilitate division of the diaphragm if needed to ensure adequate proximal control, as seen in this case. This should be done with the understanding that it can lead to later diaphragmatic hernia or pleural effusion and avoided if not necessary. In some patients, the thoracic cavity does not need to be formally entered, but do not hesitate to do so if needed. The incision is then carried obliquely across the abdomen to the lateral border of the rectus abdominis muscle and then extended inferiorly a few centimeters below the level of the umbilicus. This allows for division of the abdominal musculature without sacrifice of the rectus muscle. After dividing the transversus abdominis, the RP plane is created by bluntly retracting the peritoneum medially off of the abdominal wall. We find it easiest to develop the plane in the left lower quadrant first and identify the ureter to ensure it is not injured. This allows quick access to the iliac vessels and then the plane can be developed superiorly, lifting the kidney and spleen up and medially. At this point, ligation of the lumbar-renal vein should be performed to prevent bleeding and identify the left renal artery.
Once the medial visceral rotation is complete, an Omni retractor is placed to aid visualization and the left crus of the diaphragm is divided with cautery, exposing the supraceliac aorta. Circumferential control of the aorta at this level is obtained with an umbilical tape to facilitate complete cross clamping if an aortic complication is encountered. The tunnels can then be made bluntly into the groins. The left tunnel should be quite easy as the ureter and colon have been medialized. The right groin tunnel requires gentle dissection to identify the aortic bifurcation and then sweeping of the ureter superiorly using finger dissection facilitated by feeling the iliac vessels with the fingernail. Red rubber catheters are placed to hold the tunnels on both sides and heparin is administered.
Prior to placement of the proximal clamp, we prefer to use sterile duplex intraoperatively to ensure that the aorta is free of atheroma. In cases with posterior plaque, standard proximal and distal total clamp may be preferable and more superior dissection on the aorta facilitated by division of the diaphragm. In this case, the supraceliac aorta was normal on duplex and thus a side-biting Satinsky clamp was used for control.
Once the clamp is secured and checked to be occlusive, the aortotomy is extended with an aortic punch to remove an ellipse of tissue, and the graft is beveled and sewn end to side. The main body of the graft should be left long enough to have adequate length for the limbs to reach both femoral arteries.
After proximal anastomosis, the graft should be flushed with heparinized saline, soft clamps used, and the limbs tunneled with very little redundancy. The operation should then move to the right groin with clamping of the SFA, PA, and EIA in that order followed by longitudinal arteriotomy and endarterectomy if indicated, as in this case. The graft limb is then beveled and anastomosed end-to-side. The left femoral anastomosis is done in a similar manner, and then completion duplex of each repair is performed followed by visual and pulse exam of the feet to ensure no embolization and good hemodynamic result.
At this point, the heparin is reversed with protamine and a new coagulation panel sent to assist resuscitation and hemostasis. The groins should be left open to close last and packed with dry gauze and hemostatic agents as needed. The RP is then meticulously inspected for hemostasis and warm saline used for lavage. If the diaphragm was opened, as in this case, a chest tube is placed and then the diaphragm closed with a locking, running 0-0 monofilament suture. The ribs are reapproximated with #2 braided suture and the abdominal contents returned to the normal position. The RP incision is closed with running #1 absorbable monofilament suture and the groins closed in layers.
Useful Tips for Thoraco-femoral bypass
While division of the diaphragm is not necessary for all TFB via RP exposure, it can facilitate the visualization of the spleen as it is being mobilized and allows for more proximal aortic control. This is especially useful in male patients with a ‘barrel-chest’ body habitus from chronic smoking, as seen in this case. The diaphragm can be divided with purple load staples in an endo GIA stapler to decrease bleeding from the cut edges and facilitate closure at the end of the case. We prefer to use a locking suture technique upon closure as it prevents the suture line from becoming slack while the ribs are being reapproximated.
Visualization of the supra-celiac clamp site should be excellent as the side-biting clamp can be cumbersome to sew. Still, the physiologic effects of a partial clamp with decreased visceral ischemia outweigh surgeon inconvenience. By using an aortic punch to remove an appropriate ellipse of tissue, the proximal anastomosis can be facilitated. Even so, as seen in this case, the proximal suture line may require repair sutures. It is ideal to perform this before the clamp is removed to prevent the need to re-clamp multiple times. We prefer to test the anastomosis by injecting heparinized saline with a bulb syringe prior to clamp removal to identify obvious defects in the suture line. Once the clamp is removed, any remaining repair sutures are best performed with pledget support sutures and precise knot tying.
The advantage of intraoperative duplex evaluation cannot be overstated. We elect to perform this after the completion of the proximal anastomosis in every case to ensure no complications from clamping an adequate visceral perfusion. Pulse exam and/or continuous wave doppler alone can be deceiving. By ensuring an adequate repair, attention can be turned away from the RP incision and full attention given to performing the femoral anastomoses.
Summary
The strategy of using a left thoracoretroperitoneal aortic exposure for TFB, as seen in our patient, provides some advantages over the traditional thoracotomy technique. The major advantage to this exposure is the benefit of avoiding pulmonary related complications.18 A secondary benefit is direct access to the celiac, superior mesenteric, and left renal arteries, which can be revascularized if indicated.18 Further, this facilitates tunneling in more challenging fields as the tunnels can be accessed directly. Potential disadvantages over the traditional two cavity approach is the increased likelihood of splenic injury and higher incidence of incisional hernia.
Our patient had an uneventful hospital recovery and is now more than a year removed from the procedure with normal ABI and unlimited functional status.
In conclusion, TFB is a safe and effective treatment alternative to axillo-bifemoral bypass when patient anatomy and the extent of aortic disease burden make it less favorable. The best clinical judgment should be deferred to the operating surgeon based on their experience and the individual clinical presentation of the patient. As seen with our patient and in previously reported series, there remains a continued role for TFB in select patient populations.
Equipment
- Omni-retractor.
Disclosures
Nothing to disclose.
Statement of Consent
The patient referred to in this video article has given their informed consent to be filmed and is aware that information and images will be published online.
Note
Animation added post-publication on 05/30/2025. No changes were made to the article content.
Citations
-
Frederick M, Newman J, Kohlwes J. Leriche syndrome. J Gen Intern Med. 2010 Oct;25(10):1102-4. doi:10.1007/s11606-010-1412-z.
-
Rodríguez SP, Sandoval F. Aortoiliac occlusive disease, a silent syndrome. BMJ Case Rep. 2019 Jul 15;12(7):e230770. doi:10.1136/bcr-2019-230770.
-
Narula N, Dannenberg AJ, Olin JW, et al. Pathology of peripheral artery disease in patients with critical limb ischemia. J Am Coll Cardiol. 2018 Oct 30;72(18):2152-2163. doi:10.1016/j.jacc.2018.08.002.
-
Allison MA, Cushman M, Solomon C, et al. Ethnicity and risk factors for change in the ankle-brachial index: the Multi-Ethnic Study of Atherosclerosis. J Vasc Surg. 2009 Nov;50(5):1049-56. doi:10.1016/j.jvs.2009.05.061.
-
Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005 Oct 25;112(17):2703-7. doi:10.1161/CIRCULATIONAHA.105.546507.
-
Berger JS, Hochman J, Lobach I, Adelman MA, Riles TS, Rockman CB. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg. 2013 Sep;58(3):673-81.e1. doi:10.1016/j.jvs.2013.01.053.
-
Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004 Jan;172(1):95-105. doi:10.1016/s0021-9150(03)00204-1.
-
Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010 Jul;85(7):678-92. doi:10.4065/mcp.2010.0133.
-
Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004 Aug 10;110(6):738-43. doi:10.1161/01.CIR.0000137913.26087.F0.
-
Guirguis-Blake JM, Evans CV, Redmond N, Lin JS. Screening for peripheral artery disease using the ankle-brachial index: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018 Jul 10;320(2):184-196. doi:10.1001/jama.2018.4250.
-
Society for Vascular Surgery Lower Extremity Guidelines Writing Group; Conte MS, Pomposelli FB, Clair DG, et al; Society for Vascular Surgery. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015 Mar;61(3 Suppl):2S-41S. doi:10.1016/j.jvs.2014.12.009. Erratum in: J Vasc Surg. 2015 May;61(5):1382.
-
Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017 Mar 21;135(12):e686-e725. doi:10.1161/CIR.0000000000000470. Erratum in: Circulation. 2017 Mar 21;135(12):e790. doi:10.1161/CIR.0000000000000501.
-
Ahmed S, Raman SP, Fishman EK. CT angiography and 3D imaging in aortoiliac occlusive disease: collateral pathways in Leriche syndrome. Abdom Radiol (NY). 2017 Sep;42(9):2346-2357. doi:10.1007/s00261-017-1137-0.
-
Koelemay MJ, den Hartog D, Prins MH, Kromhout JG, Legemate DA, Jacobs MJ. Diagnosis of arterial disease of the lower extremities with duplex ultrasonography. Br J Surg. 1996 Mar;83(3):404-9. doi:10.1002/bjs.1800830336.
-
Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995 Sep 27;274(12):975-80.
-
Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996 Dec 1;94(11):3026-49. doi:10.1161/01.cir.94.11.3026. Erratum in: Circulation. 2000 Aug 29;102(9):1074.
-
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007 Jan;45 Suppl S:S5-67. doi:10.1016/j.jvs.2006.12.037.
-
Crawford JD, Scali ST, Giles KA, et al. Contemporary outcomes of thoracofemoral bypass. J Vasc Surg. 2019 Apr;69(4):1150-1159.e1. doi:10.1016/j.jvs.2018.07.053.



