Insertion of a Right-Sided PleurX Catheter for Palliation of a Malignant Pleural Effusion
Abstract
The following case describes a 91-year-old woman with no significant past medical history who presented to her primary care physician with several months of cough and progressive dyspnea. After appropriate workup she was found to have a stage IVa lung adenocarcinoma with an associated malignant pleural effusion that contributed to her symptoms. There are several therapeutic options for treating a malignant pleural effusion. An indwelling tunneled pleural catheter (PleurX catheter) is a reliable way to manage a chronic pleural effusion. The device is most commonly used to manage malignant pleural effusions, but the same technique may be applied for a range of benign, non-infectious indications as well. PleurX catheters may be inserted in an outpatient clinic, interventional radiology suite, inpatient setting, or operating room under local or general anesthesia. Once in place, they are designed to be managed in an outpatient setting either by the patient’s caregivers or by the patient themselves and serve to palliate the respiratory symptoms of a large effusion without the need for repeated thoracenteses. They can remain in place for several months, and removal in an outpatient setting with local anesthetic is trivial. Following placement of the PleurX catheter, the patient reported symptomatic improvement in her dyspnea, and she was started on dose-reduced Mobocertinib under the guidance of thoracic oncology.
Keywords
PleurX, indwelling pleural catheter, chronic pleural effusion, chronic effusion, malignant pleural effusion, malignant effusion.
Case Overview
The patient is a 91-year old woman with few other medical problems who presented to her primary care physician with a complaint of several months of cough and progressive dyspnea. Prior to this she had been living independently with minimal assistance from her children. More recently she had felt tired and had difficulty caring for herself. Her physical examination was notable for absent right-sided breath sounds. Chest x-ray demonstrated complete opacification of the right hemithorax. CT scan and subsequent PET showed a large mass in the central right hilum, which was intensely FDG avid and which obstructed the right mainstem bronchus resulting in near complete atelectasis of the right lung. The remainder of the right hemithorax was occupied by low-density fluid, and there was evidence of pleural thickening and mediastinal adenopathy. Thoracentesis was performed and 1.5 L of straw-colored fluid was removed with some improvement in the patient’s symptoms. Cytology from the pleural fluid demonstrated malignant cells with immunohistochemistry consistent with adenocarcinoma of lung origin. A diagnosis of Stage IVa lung adenocarcinoma was made. She was referred to oncology to discuss systemic treatment options and to thoracic surgery for durable palliation of the pleural effusion.
Clinical Features and Diagnosis of Malignant Pleural Effusions
A malignant pleural effusion is a feature of many advanced cancer presentations. Lung, breast, and primary mesothelial cancers are the most likely to cause a malignant pleural effusion, but any malignancy has the potential.1–3 In the normal state, the pleural space contains ~0.26 mL/kg of fluid per body kilogram mass in a constant state of production and absorption.3 A malignant effusion represents deregulation of one or both of these processes, typically by fluid production from tumor implants on the pleura or from tumor obstructing absorptive lymphatic channels.1,3
Patients present with dyspnea secondary to compressive atelectasis of the affected lung.1,4,5 Pleuritic chest pain is sometimes present and may indicate deeper chest wall invasion of metastatic pleural implants.4 Malignant effusions rarely become infected prior to instrumentation.
The diagnosis is made by combining the finding of a large effusion on imaging with a known diagnosis of advanced cancer.3,4 The fluid should be exudative based on chemistry studies but these tests are otherwise unhelpful. Fluid cytology demonstrating malignant cells is pathognomonic for the diagnosis, but negative fluid cytology does not exclude it.3,4 If there is ambiguity about the diagnosis—as frequently happens with primary mesothelioma—a diagnostic thoracoscopy with pleural biopsies should be performed.1
A malignant effusion is a sign of advanced cancer and typically portends a poor prognosis.3,5 Treatment focuses on initiating systemic therapy to control disease and palliation of the symptoms related to the pleural fluid. Interventions that minimize hospitalizations and interruptions of systemic therapy are favored over more involved, morbid procedures.3,5
In the early stage of disease it is possible to completely evacuate a malignant effusion and expand the affected lung. If the rate of pleural fluid production is not excessive, a pleural ablative procedure such as chemical or mechanical pleurodesis may provide durable palliation.1,4 Most patients present at time when entrapment of the lung by tumor and the high volume of pleural fluid precludes this approach.1 Drainage of the fluid results in a pneumothorax-ex-vacuo, characterized by air in the hemithorax coupled with an unexpanded lung. Fluid rapidly reaccumulates within this space.2 Most patients will experience some improvement in symptoms after drainage of the fluid despite incomplete lung expansion but the effect is transient.2 These patients may elect to have repeated thoracenteses, but this tends to be logistically complicated and provide poor symptom relief.3 The cumulative risk of thoracentesis also grows linearly with each subsequent attempt.
The Indwelling Tunneled Pleural Catheter as a Treatment Modality
Placement of an indwelling tunneled pleural catheter offers durable symptom relief without the need for repeated procedures.1–3,6 The catheters can be placed in the office, bedside in an inpatient setting, in an interventional suite under image guidance, or in the operating room with or without the addition of thoracoscopy.1 Once in place, the catheter can be drained on a schedule using prefabricated vacuum bottles. Nursing assistance is usually needed at first, but patients or family members frequently assume the responsibility for drainage. The process of drainage includes sterile preparation of the catheter, connection to the vacuum bottle, drainage over a period several minutes, disconnection of the catheter, and redressing of the catheter site.5,7 The volume and frequency of drainage is different depending on the patient’s circumstances. Absent other information, a recommendation for drainage of up to 1L three times a week will serve the average patient.
Advantages of an indwelling pleural catheter include durable symptom relief and fostering of patient autonomy.1,3,5 Disadvantages include the discomfort associated with placement of the catheter, discomfort associated with drainage (usually a sharp, pleuritic pain occurring late in the drainage process and indicative of a collapsing pleural space), and complications associated with the catheter itself.8 Complications include catheter dislodgement (rare after the first few weeks), catheter occlusion (salvageable by administration of transcatheter fibrinolytics), and infection of either the catheter or the pleural space. Infection is uncommon but the risk increases the longer the catheter remains.6,8 Superficial infections are treated with antibiotics and removal of the catheter, with plans for replacement at a new skin site in the near future.8 Pleural space infections are more challenging: it is often necessary to remove the catheter, place a new temporary tube for complete evacuation of the pleural space, intrapleural fibrinolysis, and intravenous antibiotics.8 Considerable time should elapse before replacement of the catheter is considered.
Technical Aspects of Placing a PleurX Catheter
In the attached video, the placement of a PleurX catheter is being performed in the operating room to demonstrate the most optimal setting and technique. The PleurX kit is self-contained and includes:
- Sterile drapes,
- Skin prep.
- Local anesthetic, syringes, and needles.
- An insertion catheter, guidewire, dilating trocar, and breakaway sheath.
- The PleurX tube and a tunneling device.
- A cap for the tube, or alternatively a connector to hook it to chest drainage system.
- Suture and dressings.
- Not included are sterile gowns and gloves and the vacuum bottles for drainage.
The partial lateral position is comfortable for patients who are able to lay flat and permits use of conscious sedation. Alternatively, patients may sit at the edge of the bed and lean over a table as they might for epidural catheter placement. Exposure of the costal arch and lateral chest wall is important. The skin is prepped and draped in sterile fashion. Optimal entry and exit points for the tube are selected. Key considerations include ease of the patient’s access and management of the tube, as well as placing the tube in a dependent position to maximize drainage. The skin is marked at the skin exit and pleural entry sites. The skin exit site is typically along the costal margin, while the pleural entry site is more posterior. A distance of 10 or more centimeters between the two sites ensures a subcutaneous tunnel that mitigates against the risk of pleural space infection; excessive distance makes the tunneling process challenging.
Local anesthetic is administered in a subcutaneous wheal at both sites. An intercostal nerve block is performed at the posterior, pleural entry site. This is performed by administering local anesthetic around the periosteum of the rib over which the catheter will be placed, entering the pleural space above this rib, drawing back pleural fluid, and then withdrawing the needle while injecting a generous amount of local anesthetic. If no pleural fluid is aspirated at this site, a new site should be selected. Ultrasound may be helpful in guiding this step.
One centimeter skin incisions are made at both sites. The tunneler is used to pass the catheter between the anterior skin exit site and the posterior pleural space entry site. At the distal end of the tube is a cuff designed to fix the tube to the subcutaneous tunnel by provoking a fibroinflammatory reaction. The cuff is ideally left just deep to the anterior skin exit incision making future removal under local anesthetic easier.
The pleural space is accessed through the posterior site using the insertion sheath. Once pleural fluid is aspirated, the needle is removed and a guidewire is placed through the sheath. The sheath is removed. A dilator is inserted over the guidewire just deep to the ribs. This is followed in series by a breakaway sheath. With the breakaway sheath in place, the wire and the inner portion of the sheath are removed and the PleurX catheter is fed through the channel. The sheath can be broken away as the catheter is advanced into the pleural space. Application of suction to the catheter with resultant drainage of pleural fluid confirms adequate placement.
The skin at the posterior pleural entry site is closed in subcutaneous fashion. The catheter may be sutured to the skin for additional security, but our preference is to use the included sterile dressings for this purpose to avoid the issue of a forgotten external suture.
The tube may be capped for later use or connected to a self-contained drainage system for complete evacuation of the pleural space over a period of time. We typically restrict drainage of fluid to no more than 1.5 L at a time to prevent the rare but potentially life-threatening complication of reexpansion pulmonary edema.
Placement of a PleurX catheter is well tolerated by patients and results in immediate improvement in those for whom pleural disease is a significant contributor to dyspnea. Marginal or absent improvement in symptoms may indicate incomplete drainage, but more likely speaks to the polyfactorial nature of dyspnea as a symptom. Complications of placement include failure to access the pleural space, subdiaphragmatic placement of the catheter (particularly in a patient with ascites), bleeding, and damage to underlying structures. Bleeding may be caused by injury to the intercostal vasculature, entry into the lung parenchyma, or disruption of pleural adhesions. Rarely, embolization or operative exploration are required.
The Patient’s Postoperative Course
Our patient tolerated the procedure well and the operation was without complication. She reported symptomatic improvement of her dyspnea within 1 week of placement of the PleurX catheter. Her catheter was drained by visiting nursing at her home for about 125–250 mL of pleural fluid twice a week for the first few weeks after placement. Subsequently the drainage output began to decrease, until the output was 0 mL about 2.5 months after placement of the PleurX catheter. About 4 months after placement, the patient was subsequently admitted to the hospital for electrolyte abnormalities, nausea, vomiting, diarrhea, and poor PO intake related to Mobocertinib systemic therapy that had been initiated by thoracic oncology. Given that the catheter output had been minimal, it was removed at the bedside during that admission.
Imaging
CT scans of the patient prior to and following PleurX catheter placement can be seen in Figure 1 and 2.
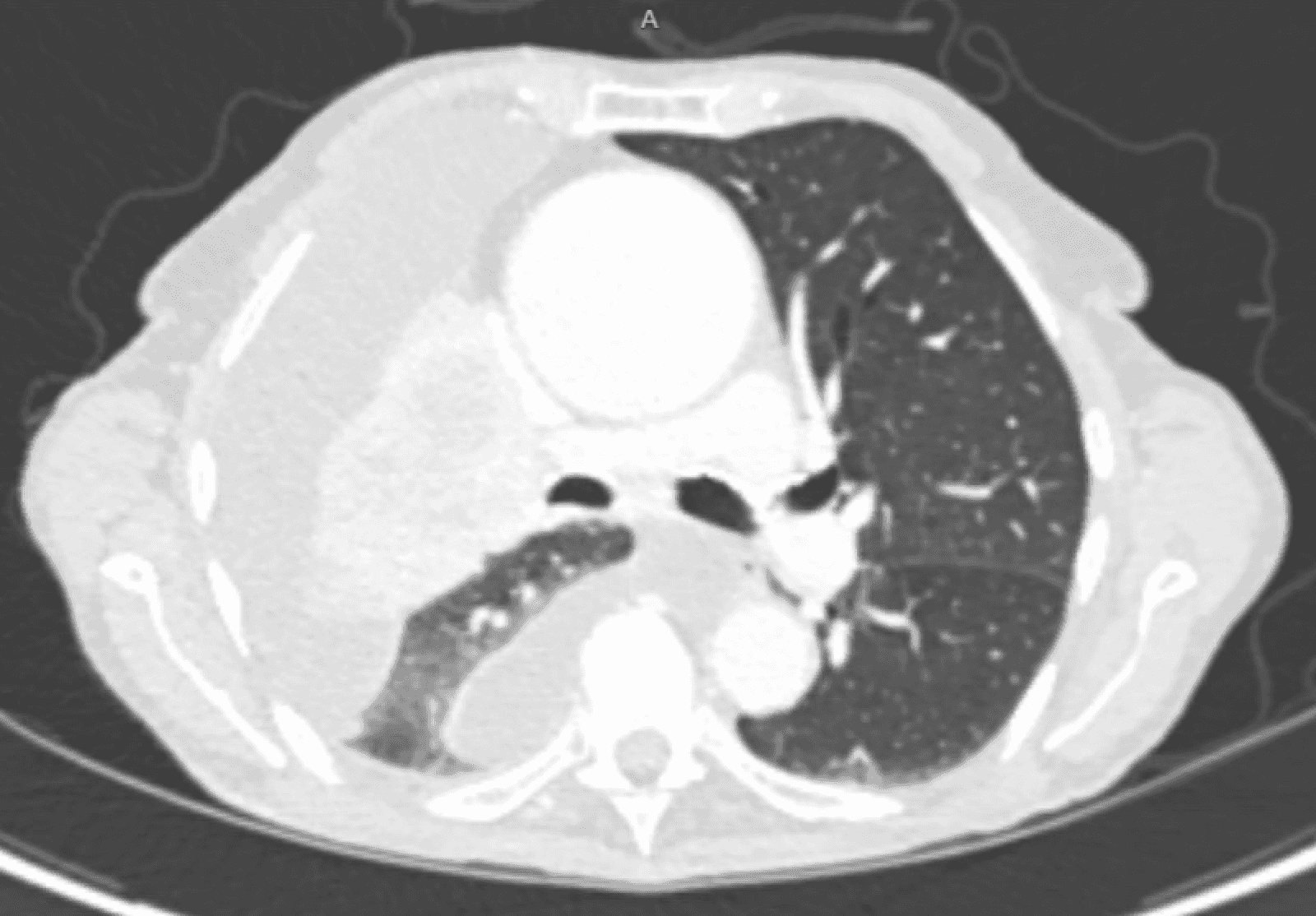
Figure 1. The CT scan of the patient prior to PleurX catheter placement.
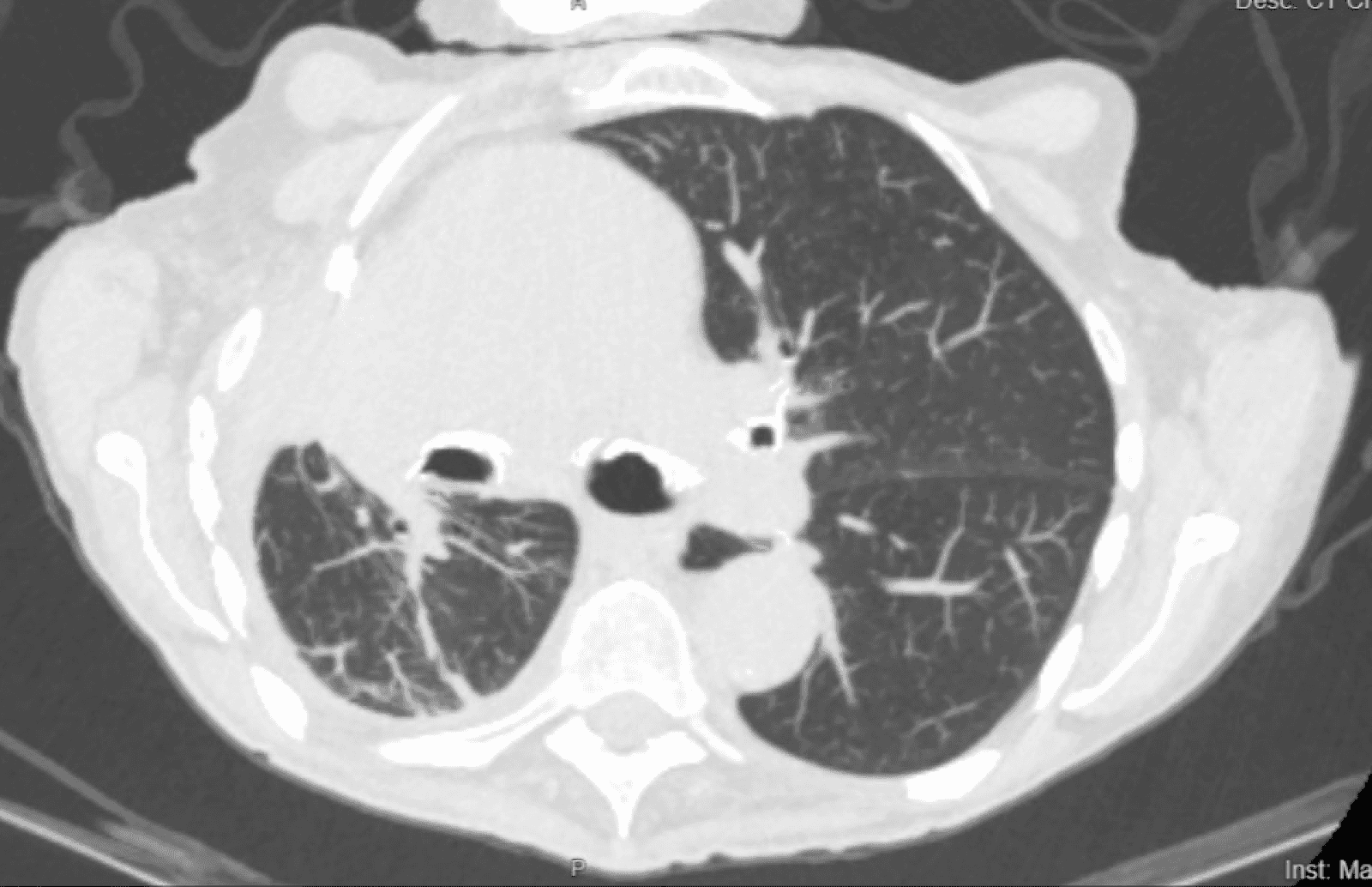
Figure 2. The CT scan of the patient 4 months following PleurX catheter placement.
Statement of Consent
The patient referred to in this video article has given their informed consent to be filmed and is aware that information and images will be published online.
Citations
-
Gonnelli F, Hassan W, Bonifazi M, et al. Malignant pleural effusion: current understanding and therapeutic approach. Respir Res. 2024 Jan 19;25(1):47. doi:10.1186/s12931-024-02684-7.
-
Hofmann HS, Scheule AM, Markowiak T, Ried M. The treatment of malignant pleural effusion with permanent indwelling pleural catheters. Dtsch Arztebl Int. 2022 Sep 5;119(35-36):595-600. doi:10.3238/arztebl.m2022.0229.
-
Skok K, Hladnik G, Grm A, Crnjac A. Malignant pleural effusion and its current management: a review. Medicina (Kaunas). 2019 Aug 15;55(8):490. doi:10.3390/medicina55080490.
-
Ferreiro L, Suárez-Antelo J, Álvarez-Dobaño JM, Toubes ME, Riveiro V, Valdés L. Malignant Pleural Effusion: Diagnosis and Management. Can Respir J. 2020 Sep 23;2020:2950751. doi:10.1155/2020/2950751.
-
Egan AM, McPhillips D, Sarkar S, Breen DP. Malignant pleural effusion. QJM. 2014 Mar;107(3):179-84. doi:10.1093/qjmed/hct245.
-
Wang S, Zhang R, Wan C, et al. Incidence of complications from indwelling pleural catheter for pleural effusion: a meta-analysis. Clin Transl Sci. 2023 Jan;16(1):104-117. doi:10.1111/cts.13430.
-
Addala DN, Kanellakis NI, Bedawi EO, Dong T, Rahman NM. Malignant pleural effusion: updates in diagnosis, management and current challenges. Front Oncol. 2022 Nov 17;12:1053574. doi:10.3389/fonc.2022.1053574.
-
Chalhoub M, Saqib A, Castellano M. Indwelling pleural catheters: complications and management strategies. J Thorac Dis. 2018 Jul;10(7):4659-4666. doi:10.21037/jtd.2018.04.160.


